Polyamine Metabolism in Leishmania Parasites: A Promising Therapeutic Target
- PMID: 35645240
- PMCID: PMC9149861
- DOI: 10.3390/medsci10020024
Polyamine Metabolism in Leishmania Parasites: A Promising Therapeutic Target
Abstract
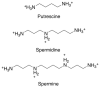
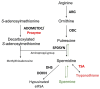
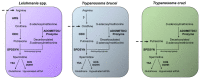
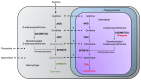
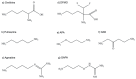
Parasites of the genus Leishmania cause a variety of devastating and often fatal diseases in humans and domestic animals worldwide. The need for new therapeutic strategies is urgent because no vaccine is available, and treatment options are limited due to a lack of specificity and the emergence of drug resistance. Polyamines are metabolites that play a central role in rapidly proliferating cells, and recent studies have highlighted their critical nature in Leishmania. Numerous studies using a variety of inhibitors as well as gene deletion mutants have elucidated the pathway and routes of transport, revealing unique aspects of polyamine metabolism in Leishmania parasites. These studies have also shed light on the significance of polyamines for parasite proliferation, infectivity, and host-parasite interactions. This comprehensive review article focuses on the main polyamine biosynthetic enzymes: ornithine decarboxylase, S-adenosylmethionine decarboxylase, and spermidine synthase, and it emphasizes recent discoveries that advance these enzymes as potential therapeutic targets against Leishmania parasites.
Keywords: Leishmania; S-adenosylmethionine decarboxylase; drug resistance; ornithine decarboxylase; polyamines; putrescine; spermidine; spermidine synthase; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization . Leishmaniasis. World Health Organization; Geneva, Switzerland: 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

