Novel Cerebello-Amygdala Connections Provide Missing Link Between Cerebellum and Limbic System
- PMID: 35645738
- PMCID: PMC9136059
- DOI: 10.3389/fnsys.2022.879634
Novel Cerebello-Amygdala Connections Provide Missing Link Between Cerebellum and Limbic System
Abstract
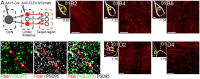
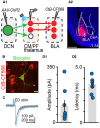
The cerebellum is emerging as a powerful regulator of cognitive and affective processing and memory in both humans and animals and has been implicated in affective disorders. How the cerebellum supports affective function remains poorly understood. The short-latency (just a few milliseconds) functional connections that were identified between the cerebellum and amygdala-a structure crucial for the processing of emotion and valence-more than four decades ago raise the exciting, yet untested, possibility that a cerebellum-amygdala pathway communicates information important for emotion. The major hurdle in rigorously testing this possibility is the lack of knowledge about the anatomy and functional connectivity of this pathway. Our initial anatomical tracing studies in mice excluded the existence of a direct monosynaptic connection between the cerebellum and amygdala. Using transneuronal tracing techniques, we have identified a novel disynaptic circuit between the cerebellar output nuclei and the basolateral amygdala. This circuit recruits the understudied intralaminar thalamus as a node. Using ex vivo optophysiology and super-resolution microscopy, we provide the first evidence for the functionality of the pathway, thus offering a missing mechanistic link between the cerebellum and amygdala. This discovery provides a connectivity blueprint between the cerebellum and a key structure of the limbic system. As such, it is the requisite first step toward obtaining new knowledge about cerebellar function in emotion, thus fundamentally advancing understanding of the neurobiology of emotion, which is perturbed in mental and autism spectrum disorders.
Keywords: anatomy; basolateral amydala; cerebellar nuclei; channelrhodopsin; circuit; electrophysiology; limbic; mouse.
Copyright © 2022 Jung, Vlasov, D’Ambra, Parigi, Baya, Frez, Villalobos, Fernandez-Frentzel, Anguiano, Ideguchi, Antzoulatos and Fioravante.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

