Chronic Ethanol Causes Persistent Increases in Alzheimer's Tau Pathology in Female 3xTg-AD Mice: A Potential Role for Lysosomal Impairment
- PMID: 35645744
- PMCID: PMC9131098
- DOI: 10.3389/fnbeh.2022.886634
Chronic Ethanol Causes Persistent Increases in Alzheimer's Tau Pathology in Female 3xTg-AD Mice: A Potential Role for Lysosomal Impairment
Abstract
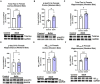
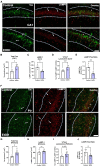
Epidemiological studies have found that heavy alcohol use is associated with increased risk for Alzheimer's disease (AD), with frequent drinking earlier in adulthood increasing risk. The increases in neuroinflammation featured in both heavy alcohol use and AD may be partially responsible for this link. However, it is unknown if abstinence mitigates this risk. We hypothesized that binge ethanol during mid adult life would persistently increase AD pathology even after prolonged abstinence. Male and female 3xTg-AD mice (APPSwe, tauP301, Psen1tm1Mpm) which feature progressive amyloid (Aβ) and tau pathology, received chronic binge ethanol (5g/kg/day, 5-days-on/2-days-off, i.g.) or water during adulthood (from 5.5 to 9 months of age), followed by abstinence and assessment at 14 months of age. The effects of ethanol on protective AD genes (e.g., APOE and TREM2) as well as proinflammatory genes were measured by PCR. Levels of pathologic tau and Aβ were measured by immunohistochemistry and western blot. Ethanol caused persistent reductions in protective AD genes: APOE (25% reduction, *p < 0.05), TREM2 (28%, *p < 0.05), LPL (40%, ** p < 0.01), and CTSD (24%, *p < 0.05) and promoted a proinflammatory gene signature in female, but not male cortex. Concurrently, ethanol increased total and hyperphosphorylated tau (AT8) in piriform cortex and hippocampus of females, but not males. Levels of AT8 were negatively correlated with APOE (R = -0.67, *p < 0.05) and TREM2 (R = -0.78, **p < 0.005) suggesting protective roles in pathogenesis. No differences were found in levels of main regulators of tau phosphorylation state (GSK3β, PKA, PP2A), suggesting ethanol disrupted clearance of tau. Therefore, we measured the effect of ethanol on lysosomes, which degrade tau, and lysosomal localization of tau using co-immunofluorescence. In females, ethanol caused a persistent reduction in mature LAMP1 lysosomes in CA1 of hippocampus (35%, *p < 0.05), along with a 60% increase in total tau (*p < 0.05). Thus, chronic binge ethanol during mid adult life causes a persistent enhancement of tau pathology in cortical and hippocampal brain regions of females. Persistent AD pathology was associated with an increased proinflammatory signature and a reduction of mature lysosomes. This implicates binge ethanol exposure with increased risk of AD pathologic progression in females.
Keywords: Alzheimer’s disease; abstinence; addiction; alcohol; neuro-inflammation; tau.
Copyright © 2022 Tucker, Alicea Pauneto, Barnett and Coleman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

