SARS-CoV-2 viral load dynamics in immunocompromised critically ill patients on remdesivir treatment
- PMID: 35646346
- PMCID: PMC9134301
- DOI: 10.4081/mrm.2022.825
SARS-CoV-2 viral load dynamics in immunocompromised critically ill patients on remdesivir treatment
Abstract
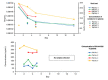
The relationship between SARS-CoV-2 quantitative viral load and risk of disease progression, morbidity such as long- COVID or mortality in immunosuppressed, remains largely undefined in COVID-19 patients. Critically ill immunosuppressed patients potentially benefit from remdesivir treatment because of the prolonged course of their infection. Four critically ill immunocompromised patients and the impact of remdesivir on viral dynamics in lower respiratory samples were studied. Bronchoalveolar lavage (BAL) samples were assessed to measure SARS-CoV-2 quantitative viral load using real-time PCR. Corresponding plasma levels of remdesivir and its metabolite GS-441524 were determined. Mean virus load of 39.74 x 107 geq/ml (±33.25 x 107 geq/ml) on day 1 dropped significantly (p<0.008) to 3.54 x 106 geq/ml (±6.93 x 106 geq/ml) on day 3 and to 1.4 x 105 geq/ml (±2.35 x 105 geq/ml) on day 5 of remdesivir treatment. Mean virus load dropped below <1% between day 1 and 5 of remdesivir treatment. Parent prodrug remdesivir and also GS441524 metabolite levels of antiviral activity in our patients were far in excess of EC 50. Our data present that remdesivir treatment potentially reduces the SARS-CoV-2 viral load in immunosuppressed critically ill patients. However, the implication of viral load reduction on morbidity and mortality needs further investigation.
Keywords: COVID-19; Remdesivir; SARS-CoV-2; immunosuppression; viral load.
©Copyright: the Author(s).
Figures
Similar articles
-
Pharmacokinetics of remdesivir and GS-441524 in two critically ill patients who recovered from COVID-19.J Antimicrob Chemother. 2020 Oct 1;75(10):2977-2980. doi: 10.1093/jac/dkaa239. J Antimicrob Chemother. 2020. PMID: 32607555 Free PMC article.
-
Comparable Efficacy of Lopinavir/Ritonavir and Remdesivir in Reducing Viral Load and Shedding Duration in Patients with COVID-19.Microorganisms. 2024 Aug 16;12(8):1696. doi: 10.3390/microorganisms12081696. Microorganisms. 2024. PMID: 39203538 Free PMC article.
-
SARS-CoV-2 Viral Load in the Pulmonary Compartment of Critically Ill COVID-19 Patients Correlates with Viral Serum Load and Fatal Outcomes.Viruses. 2022 Jun 14;14(6):1292. doi: 10.3390/v14061292. Viruses. 2022. PMID: 35746764 Free PMC article.
-
Remdesivir and its antiviral activity against COVID-19: A systematic review.Clin Epidemiol Glob Health. 2021 Jan-Mar;9:123-127. doi: 10.1016/j.cegh.2020.07.011. Epub 2020 Aug 7. Clin Epidemiol Glob Health. 2021. PMID: 32838064 Free PMC article. Review.
-
Pathogenesis-directed therapy of 2019 novel coronavirus disease.J Med Virol. 2021 Mar;93(3):1320-1342. doi: 10.1002/jmv.26610. Epub 2020 Nov 10. J Med Virol. 2021. PMID: 33073355 Review.
Cited by
-
SARS-CoV-2 viral load is linked to remdesivir efficacy in severe Covid-19 admitted to intensive care.Sci Rep. 2024 Sep 6;14(1):20825. doi: 10.1038/s41598-024-71588-9. Sci Rep. 2024. PMID: 39242658 Free PMC article.
-
Evaluating methodological approaches to assess the severity of infection with SARS-CoV-2 variants: scoping review and applications on Belgian COVID-19 data.BMC Infect Dis. 2022 Nov 11;22(1):839. doi: 10.1186/s12879-022-07777-6. BMC Infect Dis. 2022. PMID: 36368977 Free PMC article.
-
Real-life experience with remdesivir for treatment of hospitalized coronavirus disease 2019 patients: matched case-control study from a large tertiary hospital registry.Croat Med J. 2022 Dec 31;63(6):536-543. doi: 10.3325/cmj.2022.63.536. Croat Med J. 2022. PMID: 36597565 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous