Structure‒tissue exposure/selectivity relationship (STR) correlates with clinical efficacy/safety
- PMID: 35646532
- PMCID: PMC9136610
- DOI: 10.1016/j.apsb.2022.02.015
Structure‒tissue exposure/selectivity relationship (STR) correlates with clinical efficacy/safety
Abstract
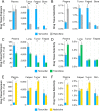
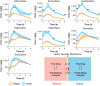
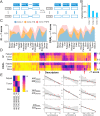
Drug optimization, which improves drug potency/specificity by structure‒activity relationship (SAR) and drug-like properties, is rigorously performed to select drug candidates for clinical trials. However, the current drug optimization may overlook the structure‒tissue exposure/selectivity-relationship (STR) in disease-targeted tissues vs. normal tissues, which may mislead the drug candidate selection and impact the balance of clinical efficacy/toxicity. In this study, we investigated the STR in correlation with observed clinical efficacy/toxicity using seven selective estrogen receptor modulators (SERMs) that have similar structures, same molecular target, and similar/different pharmacokinetics. The results showed that drug's plasma exposure was not correlated with drug's exposures in the target tissues (tumor, fat pad, bone, uterus), while tissue exposure/selectivity of SERMs was correlated with clinical efficacy/safety. Slight structure modifications of four SERMs did not change drug's plasma exposure but altered drug's tissue exposure/selectivity. Seven SERMs with high protein binding showed higher accumulation in tumors compared to surrounding normal tissues, which is likely due to tumor EPR effect of protein-bound drugs. These suggest that STR alters drug's tissue exposure/selectivity in disease-targeted tissues vs. normal tissues impacting clinical efficacy/toxicity. Drug optimization needs to balance the SAR and STR in selecting drug candidate for clinical trial to improve success of clinical drug development.
Keywords: Clinical efficacy/toxicity; Drug development; Drug optimization; Structure-tissue exposure/selectivity relationship (STR); Structure‒activity-relationship (SAR).
© 2022 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures





References
-
- Dowden H., Munro J. Trends in clinical success rates and therapeutic focus. Nat Rev Drug Discov. 2019;18:495–496. - PubMed
-
- Harrison R.K. Phase II and Phase III failures: 2013-2015. Nat Rev Drug Discov. 2016;15:817–818. - PubMed
-
- Wassermann A.M., Wawer M., Bajorath J. Activity landscape representations for structure−activity relationship analysis. J Med Chem. 2010;53:8209–8223. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

