Wnt/β-Catenin Inhibition by CWP232291 as a Novel Therapeutic Strategy in Ovarian Cancer
- PMID: 35646632
- PMCID: PMC9134752
- DOI: 10.3389/fonc.2022.852260
Wnt/β-Catenin Inhibition by CWP232291 as a Novel Therapeutic Strategy in Ovarian Cancer
Abstract
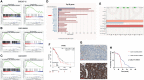
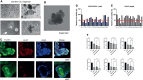
The poor prognosis of ovarian cancer patients mainly results from a lack of early diagnosis approaches and a high rate of relapse. Only a very modest improvement has been made in ovarian cancer patient survival with traditional treatments. More targeted therapies precisely matching each patient are strongly needed. The aberrant activation of Wnt/β-catenin signaling pathway plays a fundamental role in cancer development and progression in various types of cancer including ovarian cancer. Recent insight into this pathway has revealed the potential of targeting Wnt/β-catenin in ovarian cancer treatment. This study aims to investigate the effect of CWP232291, a small molecular Wnt/β-catenin inhibitor on ovarian cancer progression. Various in vitro, in vivo and ex vivo models are established for CWP232291 testing. Results show that CWP232291 could significantly attenuate ovarian cancer growth through inhibition of β-catenin. Noticeably, CWP232291 could also s suppress the growth of cisplatin-resistant cell lines and ovarian cancer patient-derived organoids. Overall, this study has firstly demonstrated the anti-tumor effect of CWP232291 in ovarian cancer and proposed Wnt/β-catenin pathway inhibition as a novel therapeutic strategy against ovarian cancer.
Keywords: CWP232291; Wnt/β-catenin; organoids; ovarian cancer; targeted therapy.
Copyright © 2022 Wang, Cho, Yoo, Jung, Kim, Kim, Lee, Jo, Han, Song, Lee, Kim, Lee, Ku, Lee and Song.
Conflict of interest statement
Authors AY and C-LJ were employed by JW Pharmaceutical Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from JW Pharmaceutical Corporation. The funder had the following involvement with the study: design and performance of the mouse experiment.
Figures

References
LinkOut - more resources
Full Text Sources

