Radiosensitivity of Breast Cancer Cells Is Dependent on the Organ Microenvironment
- PMID: 35646713
- PMCID: PMC9134193
- DOI: 10.3389/fonc.2022.833894
Radiosensitivity of Breast Cancer Cells Is Dependent on the Organ Microenvironment
Abstract
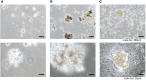
Background: Distant metastasis is the leading risk factor of death in breast cancer patients, with lung and liver being commonly involved sites of distant seeding. Ongoing clinical trials are studying the benefit from additional local treatment to these metastatic sites with radiation therapy. However, little is known about the tissue-specific microenvironment and the modulating response to treatments due to limitations of traditional in vitro systems. By using biomatrix scaffolds (BMSs) to recreate the complex composition of extracellular matrices in normal organs, we chose to study the radiotherapy response with engineered breast cancer "metastases" in liver and lung organ-specific tissues.
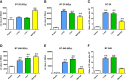
Methods: Liver and lung BMSs were prepared for tissue culture. Human breast cancer cell lines were passaged on normal tissue culture plates or tissue culture plates coated with Matrigel, liver BMSs, and lung BMSs. Clonogenic assays were performed to measure cell survival with varying doses of radiation. Reactive Oxygen Species (ROS) detection assay was used to measure ROS levels after 6 Gy irradiation to cancer cells.
Results: The response of breast cell lines to varying doses of radiotherapy is affected by their in vitro acellular microenvironment. Breast cancer cells grown in liver BMSs were more radiosensitive than when grown in lung BMSs. ROS levels for breast cancer cells cultured in lung and liver BMSs were higher than that in plastic or in Matrigel plate cells, before and after radiotherapy, highlighting the interaction with surrounding tissue-specific growth factors and cytokines. ROSs in both lung and liver BMSs were significantly increased after radiotherapy delivery, suggesting these sites create prime environments for radiation-induced cell death.
Conclusions: The therapeutic response of breast cancer metastases is dependent on the organ-specific microenvironment. The interaction between tissue microenvironment in these organs may identify sensitivity of therapeutic drug targets and radiation delivery for future studies.
Keywords: biomatrix scaffolds; breast cancer; decellularized; engineer metastases; radiation response; tumor microenvironment.
Copyright © 2022 Guo, Morse, Wang, Chen, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Separation of breast cancer and organ microenvironment transcriptomes in metastases.Breast Cancer Res. 2019 Mar 6;21(1):36. doi: 10.1186/s13058-019-1123-2. Breast Cancer Res. 2019. PMID: 30841919 Free PMC article.
-
Chromosomal in-vitro radiosensitivity of lymphocytes in radiotherapy patients and AT-homozygotes.Strahlenther Onkol. 1998 Oct;174(10):510-6. doi: 10.1007/BF03038983. Strahlenther Onkol. 1998. PMID: 9810318
-
Molecular insights into prostate cancer progression: the missing link of tumor microenvironment.J Urol. 2005 Jan;173(1):10-20. doi: 10.1097/01.ju.0000141582.15218.10. J Urol. 2005. PMID: 15592017 Review.
-
Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases.Breast Cancer Res. 2015 Mar 27;17(1):45. doi: 10.1186/s13058-015-0558-3. Breast Cancer Res. 2015. PMID: 25882816 Free PMC article.
-
All-human microphysical model of metastasis therapy.Stem Cell Res Ther. 2013;4 Suppl 1(Suppl 1):S11. doi: 10.1186/scrt372. Epub 2013 Dec 20. Stem Cell Res Ther. 2013. PMID: 24565274 Free PMC article. Review.
Cited by
-
Synthesis and Characterization of Folic Acid-Functionalized DPLA-co-PEG Nanomicelles for the Targeted Delivery of Letrozole.ACS Appl Bio Mater. 2023 May 15;6(5):1806-1815. doi: 10.1021/acsabm.3c00041. Epub 2023 Apr 24. ACS Appl Bio Mater. 2023. PMID: 37093754 Free PMC article.
References
-
- Olson R, Mathews L, Liu M, Schellenberg D, Mou B, Berrang T, et al. . Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of 1-3 Oligometastatic Tumors (SABR-COMET-3): Study Protocol for a Randomized Phase III Trial. BMC Cancer (2020) 20(1):380. doi: 10.1186/s12885-020-06876-4 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

