The Regulatory Protein ChuP Connects Heme and Siderophore-Mediated Iron Acquisition Systems Required for Chromobacterium violaceum Virulence
- PMID: 35646721
- PMCID: PMC9131926
- DOI: 10.3389/fcimb.2022.873536
The Regulatory Protein ChuP Connects Heme and Siderophore-Mediated Iron Acquisition Systems Required for Chromobacterium violaceum Virulence
Abstract
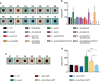
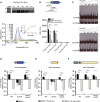
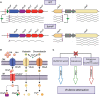
Chromobacterium violaceum is an environmental Gram-negative beta-proteobacterium that causes systemic infections in humans. C. violaceum uses siderophore-based iron acquisition systems to overcome the host-imposed iron limitation, but its capacity to use other iron sources is unknown. In this work, we characterized ChuPRSTUV as a heme utilization system employed by C. violaceum to explore an important iron reservoir in mammalian hosts, free heme and hemoproteins. We demonstrate that the chuPRSTUV genes comprise a Fur-repressed operon that is expressed under iron limitation. The chu operon potentially encodes a small regulatory protein (ChuP), an outer membrane TonB-dependent receptor (ChuR), a heme degradation enzyme (ChuS), and an inner membrane ABC transporter (ChuTUV). Our nutrition growth experiments using C. violaceum chu deletion mutants revealed that, with the exception of chuS, all genes of the chu operon are required for heme and hemoglobin utilization in C. violaceum. The mutant strains without chuP displayed increased siderophore halos on CAS plate assays. Significantly, we demonstrate that ChuP connects heme and siderophore utilization by acting as a positive regulator of chuR and vbuA, which encode the TonB-dependent receptors for the uptake of heme (ChuR) and the siderophore viobactin (VbuA). Our data favor a model of ChuP as a heme-binding post-transcriptional regulator. Moreover, our virulence data in a mice model of acute infection demonstrate that C. violaceum uses both heme and siderophore for iron acquisition during infection, with a preference for siderophores over the Chu heme utilization system.
Keywords: Chromobacterium violaceum; bacterial physiology; bacterial virulence; heme transporter; heme uptake; iron homeostasis; siderophores.
Copyright © 2022 de Lima, Batista and da Silva Neto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A quorum-sensing regulatory cascade for siderophore-mediated iron homeostasis in Chromobacterium violaceum.mSystems. 2024 Apr 16;9(4):e0139723. doi: 10.1128/msystems.01397-23. Epub 2024 Mar 19. mSystems. 2024. PMID: 38501880 Free PMC article.
-
Production and Uptake of Distinct Endogenous Catecholate-Type Siderophores Are Required for Iron Acquisition and Virulence in Chromobacterium violaceum.Infect Immun. 2019 Nov 18;87(12):e00577-19. doi: 10.1128/IAI.00577-19. Print 2019 Dec. Infect Immun. 2019. PMID: 31570563 Free PMC article.
-
Ferric Uptake Regulator Fur Coordinates Siderophore Production and Defense against Iron Toxicity and Oxidative Stress and Contributes to Virulence in Chromobacterium violaceum.Appl Environ Microbiol. 2020 Oct 15;86(21):e01620-20. doi: 10.1128/AEM.01620-20. Print 2020 Oct 15. Appl Environ Microbiol. 2020. PMID: 32859594 Free PMC article.
-
[Mechanisms and regulation of iron and heme utilization in Gram-negative bacteria].Postepy Biochem. 2005;51(2):198-208. Postepy Biochem. 2005. PMID: 16209357 Review. Polish.
-
Iron and Virulence in Stenotrophomonas Maltophilia: All We Know So Far.Front Cell Infect Microbiol. 2018 Nov 12;8:401. doi: 10.3389/fcimb.2018.00401. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30483485 Free PMC article. Review.
Cited by
-
Heme homeostasis and its regulation by hemoproteins in bacteria.mLife. 2024 Jul 11;3(3):327-342. doi: 10.1002/mlf2.12120. eCollection 2024 Sep. mLife. 2024. PMID: 39359680 Free PMC article. Review.
-
A discovery down under: decoding the draft genome sequence of Pantoea stewartii from Australia's Critically Endangered western ground parrot/kyloring (Pezoporus flaviventris).Microb Genom. 2023 Sep;9(9):001101. doi: 10.1099/mgen.0.001101. Microb Genom. 2023. PMID: 37665208 Free PMC article.
-
A quorum-sensing regulatory cascade for siderophore-mediated iron homeostasis in Chromobacterium violaceum.mSystems. 2024 Apr 16;9(4):e0139723. doi: 10.1128/msystems.01397-23. Epub 2024 Mar 19. mSystems. 2024. PMID: 38501880 Free PMC article.
-
Bradyrhizobium japonicum HmuP is an RNA-binding protein that positively controls hmuR operon expression by suppression of a negative regulatory RNA element in the 5' untranslated region.Mol Microbiol. 2024 Jun;121(6):1217-1227. doi: 10.1111/mmi.15274. Epub 2024 May 9. Mol Microbiol. 2024. PMID: 38725184 Free PMC article.
-
Sepsis‑induced cardiac dysfunction and pathogenetic mechanisms (Review).Mol Med Rep. 2023 Dec;28(6):227. doi: 10.3892/mmr.2023.13114. Epub 2023 Oct 20. Mol Med Rep. 2023. PMID: 37859613 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

