Multifunctional Anti-Alzheimer's Disease Effects of Natural Xanthone Derivatives: A Primary Structure-Activity Evaluation
- PMID: 35646819
- PMCID: PMC9130743
- DOI: 10.3389/fchem.2022.842208
Multifunctional Anti-Alzheimer's Disease Effects of Natural Xanthone Derivatives: A Primary Structure-Activity Evaluation
Abstract
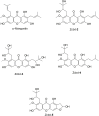
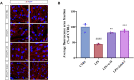
Background: A series of α-Mangostin (α-M) derivatives were designed and synthesized. α-M and four analogues were evaluated for their multifunctional anti-Alzheimer's disease (anti-AD) effects on fibrillogenesis, microglial uptake, microglial degradation, and anti-neurotoxicity of Aβ, as well as LPS-induced neuroinflammation. The differences in bioactivities were analyzed to understand the structure-activity relationship for further modifications. Purpose: This study aims to investigate the anti-AD effects of α-M and elucidate its structure-activity relationship by comparing difference between α-M and several analogues. Methods: Aβ fibrillogenesis was detected by Thioflavin T fluorometric assay. The levels of Aβ1-42 and inflammatory cytokines were evaluated by enzyme-linked immunosorbent assay. Neuron viability was examined by the CCK-8 assay. The morphology of ZO-1 of bEnd.3 cultured in BV-2-conditioned medium was evaluated by immunofluorescence staining. Results: Aβ fibrillogenesis was significantly inhibited by co-incubation with α-M, Zcbd-2 or Zcbd-3. α-M, Zcbd-2, Zcbd-3, and Zcbd-4 decreased the levels of Aβ1-42 and inflammatory cytokines, and promoted Aβ uptake, degradation and anti-inflammation effects inflammation in microglia. α-M and Zcbd-3 protected neuron viability from Aβ-induced neurotoxicity, and preserved tight junction integrity of bEnd.3 against LPS-induced neuroinflammation. Conclusion: Zcbd-3 acted as α-M almost in all effects. The structure-activity analysis indicated that the 3-methyl-2-butenyl group at C-8 is essential for the bioactivity of α-M, while modifying the double hydroxylation at the C-2 position may improve the multifunctional anti-AD effects.
Keywords: Alzheimer’s disease; amyloid beta; multifunctional; neuroinflammation; structure-activity; α-mangostin.
Copyright © 2022 Hu, Liu, Wang, Zhao, Qiu, Chen, Hu and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

