Deciphering the Structure and Formation of Amyloids in Neurodegenerative Diseases With Chemical Biology Tools
- PMID: 35646824
- PMCID: PMC9133342
- DOI: 10.3389/fchem.2022.886382
Deciphering the Structure and Formation of Amyloids in Neurodegenerative Diseases With Chemical Biology Tools
Abstract
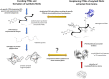
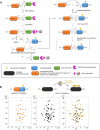
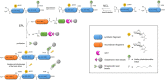
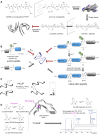
Protein aggregation into highly ordered, regularly repeated cross-β sheet structures called amyloid fibrils is closely associated to human disorders such as neurodegenerative diseases including Alzheimer's and Parkinson's diseases, or systemic diseases like type II diabetes. Yet, in some cases, such as the HET-s prion, amyloids have biological functions. High-resolution structures of amyloids fibrils from cryo-electron microscopy have very recently highlighted their ultrastructural organization and polymorphisms. However, the molecular mechanisms and the role of co-factors (posttranslational modifications, non-proteinaceous components and other proteins) acting on the fibril formation are still poorly understood. Whether amyloid fibrils play a toxic or protective role in the pathogenesis of neurodegenerative diseases remains to be elucidated. Furthermore, such aberrant protein-protein interactions challenge the search of small-molecule drugs or immunotherapy approaches targeting amyloid formation. In this review, we describe how chemical biology tools contribute to new insights on the mode of action of amyloidogenic proteins and peptides, defining their structural signature and aggregation pathways by capturing their molecular details and conformational heterogeneity. Challenging the imagination of scientists, this constantly expanding field provides crucial tools to unravel mechanistic detail of amyloid formation such as semisynthetic proteins and small-molecule sensors of conformational changes and/or aggregation. Protein engineering methods and bioorthogonal chemistry for the introduction of protein chemical modifications are additional fruitful strategies to tackle the challenge of understanding amyloid formation.
Keywords: aggregation; amyloid fibril; fluorescent probes; nanobody; native chemical ligation; neurodegenerative diseases; posttranslational modifications; protein semisynthesis.
Copyright © 2022 Landrieu, Dupré, Sinnaeve, El Hajjar and Smet-Nocca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
Publication types
LinkOut - more resources
Full Text Sources

