Comparative Proteomics Reveals Evidence of Enhanced EPA Trafficking in a Mutant Strain of Nannochloropsis oculata
- PMID: 35646838
- PMCID: PMC9134194
- DOI: 10.3389/fbioe.2022.838445
Comparative Proteomics Reveals Evidence of Enhanced EPA Trafficking in a Mutant Strain of Nannochloropsis oculata
Abstract
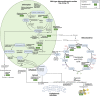
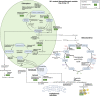
The marine microalga Nannochloropsis oculata is a bioproducer of eicosapentaenoic acid (EPA), a fatty acid. EPA is incorporated into monogalactosyldiacylglycerol within N. oculata thylakoid membranes, and there is a biotechnological need to remodel EPA synthesis to maximize production and simplify downstream processing. In this study, random mutagenesis and chemical inhibitor-based selection method were devised to increase EPA production and accessibility for improved extraction. Ethyl methanesulfonate was used as the mutagen with selective pressure achieved by using two enzyme inhibitors of lipid metabolism: cerulenin and galvestine-1. Fatty acid methyl ester analysis of a selected fast-growing mutant strain had a higher percentage of EPA (37.5% of total fatty acids) than the wild-type strain (22.2% total fatty acids), with the highest EPA quantity recorded at 68.5 mg/g dry cell weight, while wild-type cells had 48.6 mg/g dry cell weight. Label-free quantitative proteomics for differential protein expression analysis revealed that the wild-type and mutant strains might have alternative channeling pathways for EPA synthesis. The mutant strain showed potentially improved photosynthetic efficiency, thus synthesizing a higher quantity of membrane lipids and EPA. The EPA synthesis pathways could also have deviated in the mutant, where fatty acid desaturase type 2 (13.7-fold upregulated) and lipid droplet surface protein (LDSP) (34.8-fold upregulated) were expressed significantly higher than in the wild-type strain. This study increases the understanding of EPA trafficking in N. oculata, leading to further strategies that can be implemented to enhance EPA synthesis in marine microalgae.
Keywords: Nannochloropsis; cerulenin; eicosapentaenoic acid (EPA); ethyl methanesulfonate (EMS) random mutagenesis; galvestine-1; label-free quantitative (LFQ) proteomic analysis.
Copyright © 2022 Wan Razali, Evans and Pandhal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Combining high pressure and electric fields towards Nannochloropsis oculata eicosapentaenoic acid-rich extracts.Appl Microbiol Biotechnol. 2023 Aug;107(16):5063-5077. doi: 10.1007/s00253-023-12626-w. Epub 2023 Jun 29. Appl Microbiol Biotechnol. 2023. PMID: 37382612 Free PMC article.
-
Production of eicosapentaenoic acid by Nannochloropsis oculata: Effects of carbon dioxide and glycerol.J Biotechnol. 2016 Dec 10;239:47-56. doi: 10.1016/j.jbiotec.2016.10.006. Epub 2016 Oct 8. J Biotechnol. 2016. PMID: 27725210
-
Astaxanthin and eicosapentaenoic acid production by S4, a new mutant strain of Nannochloropsis gaditana.Microb Cell Fact. 2022 Jun 16;21(1):117. doi: 10.1186/s12934-022-01847-9. Microb Cell Fact. 2022. PMID: 35710482 Free PMC article.
-
Lipid Production from Nannochloropsis.Mar Drugs. 2016 Mar 25;14(4):61. doi: 10.3390/md14040061. Mar Drugs. 2016. PMID: 27023568 Free PMC article. Review.
-
A biorefinery for Nannochloropsis: Induction, harvesting, and extraction of EPA-rich oil and high-value protein.Bioresour Technol. 2017 Nov;244(Pt 2):1416-1424. doi: 10.1016/j.biortech.2017.05.124. Epub 2017 May 21. Bioresour Technol. 2017. PMID: 28624245 Review.
Cited by
-
Nannochloropsis Lipids and Polyunsaturated Fatty Acids: Potential Applications and Strain Improvement.Mar Drugs. 2025 Mar 15;23(3):128. doi: 10.3390/md23030128. Mar Drugs. 2025. PMID: 40137314 Free PMC article. Review.
-
Marine Microalgae-Microorganism Co-Cultures: An Insight into Nannochloropsis sp. Use and Biotechnological Applications.Foods. 2025 Apr 26;14(9):1522. doi: 10.3390/foods14091522. Foods. 2025. PMID: 40361605 Free PMC article. Review.
-
Microalgae biofuels: illuminating the path to a sustainable future amidst challenges and opportunities.Biotechnol Biofuels Bioprod. 2024 Jan 23;17(1):10. doi: 10.1186/s13068-024-02461-0. Biotechnol Biofuels Bioprod. 2024. PMID: 38254224 Free PMC article. Review.
-
LFQRatio: A Normalization Method to Decipher Quantitative Proteome Changes in Microbial Coculture Systems.J Proteome Res. 2024 Mar 1;23(3):999-1013. doi: 10.1021/acs.jproteome.3c00714. Epub 2024 Feb 14. J Proteome Res. 2024. PMID: 38354288 Free PMC article.
References
-
- Arora N., Yen H.-W., Philippidis G. P. (2020). Harnessing the Power of Mutagenesis and Adaptive Laboratory Evolution for High Lipid Production by Oleaginous Microalgae and Yeasts. Sustainability 12, 5125. 10.3390/su12125125 - DOI
-
- Babuskin S., Krishnan K. R., Babu P. A. S., Sivarajan M., Sukumar M. (2014). Functional Foods Enriched with marine Microalga Nannochloropsis Oculata as a Source of ω-3 Fatty Acids. Food Technol. Biotechnol. 52, 292–299.
LinkOut - more resources
Full Text Sources
Research Materials

