Thyroid Hormones Deficiency Impairs Male Germ Cell Development: A Cross Talk Between Hypothalamic-Pituitary-Thyroid, and-Gonadal Axes in Zebrafish
- PMID: 35646887
- PMCID: PMC9133415
- DOI: 10.3389/fcell.2022.865948
Thyroid Hormones Deficiency Impairs Male Germ Cell Development: A Cross Talk Between Hypothalamic-Pituitary-Thyroid, and-Gonadal Axes in Zebrafish
Abstract
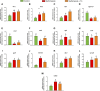
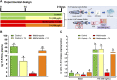
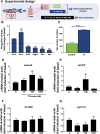
In vertebrates, thyroid hormones are critical players in controlling different physiological processes such as development, growth, metabolism among others. There is evidence in mammals that thyroid hormones are also an important component of the hormonal system that controls reproduction, although studies in fish remain poorly investigated. Here, we tested this hypothesis by investigating the effects of methimazole-induced hypothyroidism on the testicular function in adult zebrafish. Treatment of fish with methimazole, in vivo, significantly altered zebrafish spermatogenesis by inhibiting cell differentiation and meiosis, as well as decreasing the relative number of spermatozoa. The observed impairment of spermatogenesis by methimazole was correlated with significant changes in transcript levels for several genes implicated in the control of reproduction. Using an in vitro approach, we also demonstrated that in addition to affecting the components of the brain-pituitary-peripheral axis, T3 (triiodothyronine) also exerts direct action on the testis. These results reinforce the hypothesis that thyroid hormones are an essential element of multifactorial control of reproduction and testicular function in zebrafish and possibly other vertebrate species.
Keywords: germ cell; hypothyroidism; methimazole; spermatogenesis; thyroid hormones; zebrafish.
Copyright © 2022 Rodrigues, Tovo-Neto, Rosa, Doretto, Fallah, Habibi and Nóbrega.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Interaction between thyroid hormones and gonadotropin inhibitory hormone in ex vivo culture of zebrafish testis: An approach to study multifactorial control of spermatogenesis.Mol Cell Endocrinol. 2021 Jul 15;532:111331. doi: 10.1016/j.mce.2021.111331. Epub 2021 May 24. Mol Cell Endocrinol. 2021. PMID: 34038752
-
Thyroid hormone actions on male reproductive system of teleost fish.Gen Comp Endocrinol. 2018 Sep 1;265:230-236. doi: 10.1016/j.ygcen.2018.04.023. Epub 2018 Apr 17. Gen Comp Endocrinol. 2018. PMID: 29678724 Review.
-
Hypothalamic-Pituitary-Thyroid Axis Crosstalk With the Hypothalamic-Pituitary-Gonadal Axis and Metabolic Regulation in the Eurasian Tree Sparrow During Mating and Non-mating Periods.Front Endocrinol (Lausanne). 2020 May 29;11:303. doi: 10.3389/fendo.2020.00303. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32547486 Free PMC article.
-
Cortisol Directly Stimulates Spermatogonial Differentiation, Meiosis, and Spermiogenesis in Zebrafish (Danio rerio) Testicular Explants.Biomolecules. 2020 Mar 10;10(3):429. doi: 10.3390/biom10030429. Biomolecules. 2020. PMID: 32164184 Free PMC article.
-
The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys.Gen Comp Endocrinol. 2009 Mar;161(1):20-9. doi: 10.1016/j.ygcen.2008.11.023. Epub 2008 Dec 3. Gen Comp Endocrinol. 2009. PMID: 19084529 Review.
Cited by
-
Methimazole-induced reproductive endocrine system disruption and hepatotoxicity: insights from a Trichogaster trichopterus animal model.BMC Pharmacol Toxicol. 2025 Jul 1;26(1):128. doi: 10.1186/s40360-025-00942-y. BMC Pharmacol Toxicol. 2025. PMID: 40598691 Free PMC article.
-
The Role of Neurohypophysial Hormones in the Endocrine and Paracrine Control of Gametogenesis in Fish.Cells. 2025 Jul 10;14(14):1061. doi: 10.3390/cells14141061. Cells. 2025. PMID: 40710314 Free PMC article. Review.
-
Thyroid axis participates in high-temperature-induced male sex reversal through its activation by the stress response.Cell Mol Life Sci. 2023 Aug 17;80(9):253. doi: 10.1007/s00018-023-04913-6. Cell Mol Life Sci. 2023. PMID: 37589787 Free PMC article.
-
Roles of thyroid and leptin hormones and their crosstalk in male reproductive functions: an updated review.Endocrine. 2025 Mar;87(3):891-906. doi: 10.1007/s12020-024-04069-8. Epub 2024 Oct 16. Endocrine. 2025. PMID: 39412610 Review.
-
Experimentally Manipulating the Thyroid Hormone Axis in Zebrafish.Methods Mol Biol. 2025;2876:189-198. doi: 10.1007/978-1-0716-4252-8_13. Methods Mol Biol. 2025. PMID: 39579317 Review.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

