Emerging Role of Ubiquitin-Specific Protease 19 in Oncogenesis and Cancer Development
- PMID: 35646888
- PMCID: PMC9133600
- DOI: 10.3389/fcell.2022.889166
Emerging Role of Ubiquitin-Specific Protease 19 in Oncogenesis and Cancer Development
Abstract
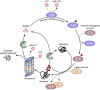
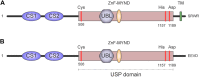
Ubiquitination and ubiquitin-like post-translational modifications control the activity and stability of different tumor suppressors and oncoproteins. Hence, regulation of this enzymatic cascade offers an appealing scenario for novel antineoplastic targets discovery. Among the different families of enzymes that participate in the conjugation of Ubiquitin, deubiquitinating enzymes (DUBs), responsible for removing ubiquitin or ubiquitin-like peptides from substrate proteins, have attracted increasing attention. In this regard, increasing evidence is accumulating suggesting that the modulation of the catalytic activity of DUBs represents an attractive point of therapeutic intervention in cancer treatment. In particular, different lines of research indicate that USP19, a member of the DUBs, plays a role in the control of tumorigenesis and cancer dissemination. This review aims at summarizing the current knowledge of USP19 wide association with the control of several cellular processes in different neoplasms, which highlights the emerging role of USP19 as a previously unrecognized prognosis factor that possesses both positive and negative regulation activities in tumor biology. These observations indicate that USP19 might represent a novel putative pharmacologic target in oncology and underscores the potential of identifying specific modulators to test in clinical settings.
Keywords: DUBs; PTMs; USP19; metastasis; tumorigenesis; ubiquitination.
Copyright © 2022 Rossi and Rossi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Avvakumov G. V., Walker J. R., Xue S., Finerty P. J., Mackenzie F., Newman E. M., et al. (2006). Amino-terminal Dimerization, NRDP1-Rhodanese Interaction, and Inhibited Catalytic Domain Conformation of the Ubiquitin-specific Protease 8 (USP8). J. Biol. Chem. 281, 38061. 10.1074/jbc.m606704200 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

