When PIP2 Meets p53: Nuclear Phosphoinositide Signaling in the DNA Damage Response
- PMID: 35646908
- PMCID: PMC9136457
- DOI: 10.3389/fcell.2022.903994
When PIP2 Meets p53: Nuclear Phosphoinositide Signaling in the DNA Damage Response
Abstract
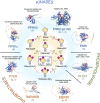
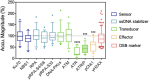
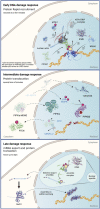
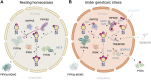
The mechanisms that maintain genome stability are critical for preventing tumor progression. In the past decades, many strategies were developed for cancer treatment to disrupt the DNA repair machinery or alter repair pathway selection. Evidence indicates that alterations in nuclear phosphoinositide lipids occur rapidly in response to genotoxic stresses. This implies that nuclear phosphoinositides are an upstream element involved in DNA damage signaling. Phosphoinositides constitute a new signaling interface for DNA repair pathway selection and hence a new opportunity for developing cancer treatment strategies. However, our understanding of the underlying mechanisms by which nuclear phosphoinositides regulate DNA damage repair, and particularly the dynamics of those processes, is rather limited. This is partly because there are a limited number of techniques that can monitor changes in the location and/or abundance of nuclear phosphoinositide lipids in real time and in live cells. This review summarizes our current knowledge regarding the roles of nuclear phosphoinositides in DNA damage response with an emphasis on the dynamics of these processes. Based upon recent findings, there is a novel model for p53's role with nuclear phosphoinositides in DNA damage response that provides new targets for synthetic lethality of tumors.
Keywords: DNA damage response (DDR); DNA repair; nuclear phosphoinositide signaling; p53; repair pathway choice; stress response.
Copyright © 2022 Wang and Sheetz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Alvarez-Venegas R., Sadder M., Hlavacka A., Baluška F., Xia Y., Lu G., et al. (2006). The Arabidopsis Homolog of Trithorax, ATX1, Binds Phosphatidylinositol 5-phosphate, and the Two Regulate a Common Set of Target Genes. Proc. Natl. Acad. Sci. U.S.A. 103, 6049–6054. 10.1073/pnas.0600944103 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

