Spatial Learning Promotes Adult Neurogenesis in Specific Regions of the Zebrafish Pallium
- PMID: 35646912
- PMCID: PMC9130729
- DOI: 10.3389/fcell.2022.840964
Spatial Learning Promotes Adult Neurogenesis in Specific Regions of the Zebrafish Pallium
Abstract
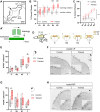
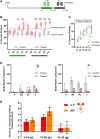
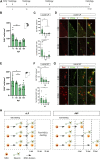
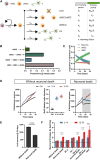
Adult neurogenesis could be considered as a homeostatic mechanism that accompanies the continuous growth of teleost fish. As an alternative but not excluding hypothesis, adult neurogenesis would provide a form of plasticity necessary to adapt the brain to environmental challenges. The zebrafish pallium is a brain structure involved in the processing of various cognitive functions and exhibits extended neurogenic niches throughout the periventricular zone. The involvement of neuronal addition as a learning-related plastic mechanism has not been explored in this model, yet. In this work, we trained adult zebrafish in a spatial behavioral paradigm and evaluated the neurogenic dynamics in different pallial niches. We found that adult zebrafish improved their performance in a cue-guided rhomboid maze throughout five daily sessions, being the fish able to relearn the task after a rule change. This cognitive activity increased cell proliferation exclusively in two pallial regions: the caudal lateral pallium (cLP) and the rostral medial pallium (rMP). To assessed whether learning impinges on pallial adult neurogenesis, mitotic cells were labeled by BrdU administration, and then fish were trained at different periods of adult-born neuron maturation. Our results indicate that adult-born neurons are being produced on demand in rMP and cLP during the learning process, but with distinct critical periods among these regions. Next, we evaluated the time course of adult neurogenesis by pulse and chase experiments. We found that labeled cells decreased between 4 and 32 dpl in both learning-sensitive regions, whereas a fraction of them continues proliferating over time. By modeling the population dynamics of neural stem cells (NSC), we propose that learning increases adult neurogenesis by two mechanisms: driving a chained proliferation of labeled NSC and rescuing newborn neurons from death. Our findings highlight adult neurogenesis as a conserved source of brain plasticity and shed light on a rostro-caudal specialization of pallial neurogenic niches in adult zebrafish.
Keywords: danio rerio (zebrafish); neural stem/progenitor cells; plasticity; spatial learning and memory; telencephalon.
Copyright © 2022 Mazzitelli-Fuentes, Román, Castillo Elías, Deleglise and Mongiat.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Life-Long Neurogenic Activity of Individual Neural Stem Cells and Continuous Growth Establish an Outside-In Architecture in the Teleost Pallium.Curr Biol. 2017 Nov 6;27(21):3288-3301.e3. doi: 10.1016/j.cub.2017.09.052. Epub 2017 Oct 26. Curr Biol. 2017. PMID: 29107546 Free PMC article.
-
Mechanical Brain Injury Increases Cells' Production of Cystathionine β-Synthase and Glutamine Synthetase, but Reduces Pax2 Expression in the Telencephalon of Juvenile Chum Salmon, Oncorhynchus keta.Int J Mol Sci. 2021 Jan 28;22(3):1279. doi: 10.3390/ijms22031279. Int J Mol Sci. 2021. PMID: 33525421 Free PMC article.
-
Sensory-specific modulation of adult neurogenesis in sensory structures is associated with the type of stem cell present in the neurogenic niche of the zebrafish brain.Eur J Neurosci. 2014 Dec;40(11):3591-607. doi: 10.1111/ejn.12729. Epub 2014 Sep 18. Eur J Neurosci. 2014. PMID: 25231569
-
Conserved and Divergent Features of Adult Neurogenesis in Zebrafish.Front Cell Dev Biol. 2020 Jun 30;8:525. doi: 10.3389/fcell.2020.00525. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32695781 Free PMC article. Review.
-
Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration.Int J Mol Sci. 2024 Mar 25;25(7):3658. doi: 10.3390/ijms25073658. Int J Mol Sci. 2024. PMID: 38612470 Free PMC article. Review.
Cited by
-
11β-Hydroxysteroid dehydrogenase type 2 may mediate the stress-specific effects of cortisol on brain cell proliferation in adult zebrafish (Danio rerio).J Exp Biol. 2024 Aug 15;227(16):jeb248020. doi: 10.1242/jeb.248020. Epub 2024 Aug 26. J Exp Biol. 2024. PMID: 39092490 Free PMC article.
-
Learning and memory formation in zebrafish: Protein dynamics and molecular tools.Front Cell Dev Biol. 2023 Mar 9;11:1120984. doi: 10.3389/fcell.2023.1120984. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36968211 Free PMC article. Review.
-
A Critical Review of Zebrafish Neurological Disease Models-1. The Premise: Neuroanatomical, Cellular and Genetic Homology and Experimental Tractability.Oxf Open Neurosci. 2023 Jan 6;2:kvac018. doi: 10.1093/oons/kvac018. eCollection 2023. Oxf Open Neurosci. 2023. PMID: 37649777 Free PMC article.
-
Constitutive Neurogenesis and Neuronal Plasticity in the Adult Cerebellum and Brainstem of Rainbow Trout, Oncorhynchus mykiss.Int J Mol Sci. 2024 May 21;25(11):5595. doi: 10.3390/ijms25115595. Int J Mol Sci. 2024. PMID: 38891784 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

