Sequence Determinants of TDP-43 Ribonucleoprotein Condensate Formation and Axonal Transport in Neurons
- PMID: 35646935
- PMCID: PMC9133736
- DOI: 10.3389/fcell.2022.876893
Sequence Determinants of TDP-43 Ribonucleoprotein Condensate Formation and Axonal Transport in Neurons
Abstract
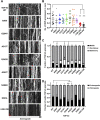
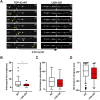
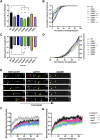
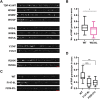
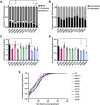
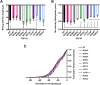
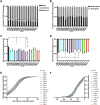
Mutations in TDP-43, a RNA-binding protein with multiple functions in RNA metabolism, cause amyotrophic lateral sclerosis (ALS), but it is uncertain how defects in RNA biology trigger motor neuron degeneration. TDP-43 is a major constituent of ribonucleoprotein (RNP) granules, phase separated biomolecular condensates that regulate RNA splicing, mRNA transport, and translation. ALS-associated TDP-43 mutations, most of which are found in the low complexity domain, promote aberrant liquid to solid phase transitions and impair the dynamic liquid-like properties and motility of RNP transport granules in neurons. Here, we perform a comparative analysis of ALS-linked mutations and TDP-43 variants in order to identify critical structural elements, aromatic and charged residues that are key determinants of TDP-43 RNP transport and condensate formation in neurons. We find that A315T and Q343R disease-linked mutations and substitutions of aromatic residues within the α-helical domain and LARKS, show the most severe defects in TDP-43 RNP granule transport and impair both anterograde and retrograde motility. F313L and F313-6L/Y substitutions of one or both phenylalanine residues in LARKS suggest the aromatic rings are important for TDP-43 RNP transport. Similarly, W334F/L substitutions of the tryptophan residue in the α-helical domain, impair TDP-43 RNP motility (W334L) or anterograde transport (W334F). We also show that R293A and R293K mutations, which disrupt the only RGG in the LCD, profoundly reduce long-range, directed transport and net velocity of TDP-43 RNP granules. In the disordered regions flanking the α-helical domain, we find that F283Y, F397Y or Y374F substitutions of conserved GF/G and SYS motifs, also impair anterograde and/or retrograde motility, possibly by altering hydrophobicity. Similarly, ALS-linked mutations in disordered regions distant from the α-helical domain also show anterograde transport deficits, consistent with previous findings, but these mutations are less severe than A315T and Q343R. Overall our findings demonstrate that the conserved α-helical domain, phenylalanine residues within LARKS and RGG motif are key determinants of TDP-43 RNP transport, suggesting they may mediate efficient recruitment of motors and adaptor proteins. These results offer a possible mechanism underlying ALS-linked TDP-43 defects in axonal transport and homeostasis.
Keywords: TDP-43; amyotrophic lateral sclerosis; axonal transport; biomolecular condensates; neuron; ribonucleoprotein granules.
Copyright © 2022 Vishal, Wijegunawardana, Salaikumaran and Gopal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Altman T., Ionescu A., Ibraheem A., Priesmann D., Gradus-Pery T., Farberov L., et al. (2021). Axonal TDP-43 Condensates Drive Neuromuscular Junction Disruption through Inhibition of Local Synthesis of Nuclear Encoded Mitochondrial Proteins. Nat. Commun. 12 (1), 6914. 10.1038/s41467-021-27221-8 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

