Protocol for a Sepsis Model Utilizing Fecal Suspension in Mice: Fecal Suspension Intraperitoneal Injection Model
- PMID: 35646946
- PMCID: PMC9134078
- DOI: 10.3389/fmed.2022.765805
Protocol for a Sepsis Model Utilizing Fecal Suspension in Mice: Fecal Suspension Intraperitoneal Injection Model
Abstract
Background: Various animal models of sepsis have been developed to optimize sepsis treatment. However, therapeutic agents that were successful in animal models were rarely effective in human clinical trials. The cecal ligation and puncture (CLP) model is currently the gold standard for sepsis studies. However, its limitations include the high variability among researchers and the difficulty in comparing animals with different cecum shapes and sizes. In this study, we established a protocol for the creation of a simple and accessible sepsis rodent model using fecal suspensions that minimized differences in technical effects among researchers and individual differences in animals.
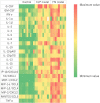
Methods: A mouse model of sepsis using fecal suspension intraperitoneal injection (FSI) was created using fresh stool excreted within 24 h. The collected fresh stool was dissolved in saline solution and filtered. The obtained fecal suspension was injected intraperitoneally into the mice. Moreover, fecal suspensions with different concentrations were prepared, and the survival rates were compared among the fecal suspensions for each concentration. To assess the validity of the FSI as a sepsis model, CLP and FSI with similar mortality rates were compared pathologically, physiologically, immunologically, and bacteriologically. Histopathological comparison was evaluated by hematoxylin-eosin and Gram staining of the parenchymal organs. Physiological evaluation was performed by comparing the respiratory rate, body temperature, and blood gas analysis results. Immunological assessment was performed using multiplex analysis. Bacteriological comparisons were performed by culturing ascites fluid.
Results: The FSI model increased mortality in proportion to the fecal suspension concentration. The mortality rate was reduced with antibiotic administration. In various comparative experiments conducted using the FSI and CLP models, both models showed findings consistent with sepsis. Furthermore, the FSI model showed less variability among the individuals in each test.
Conclusion: This is the first detailed and accurate report of a protocol for creating a sepsis model using fecal suspension. The FSI model is a minimally invasive and accessible sepsis rodent model. Its clinical validity as a sepsis model was proven via histological, physiological, microbiological, and immunological evaluation methods. The FSI model minimizes individual differences between mice and helps to conduct accurate studies after the onset of sepsis.
Keywords: cecal ligation and puncture; cytokine; inflammation; mice; peritonitis; sepsis.
Copyright © 2022 Tsuchida, Wada, Mizugaki, Oda, Kayano, Yamakawa and Tanaka.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab’)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. (2004) 32:2173–82. 10.1097/01.ccm.0000145229.59014.6c - DOI - PubMed
-
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, III, Bland KI, et al. Cecal ligation and puncture. Shock. (2005) 24(Suppl. 1):52–7. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

