Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways
- PMID: 35646963
- PMCID: PMC9131003
- DOI: 10.3389/fmed.2022.837222
Advanced Glycation End Products in the Skin: Molecular Mechanisms, Methods of Measurement, and Inhibitory Pathways
Abstract
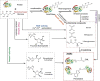
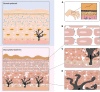
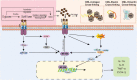
Advanced glycation end products (AGEs) are a series of stable compounds produced under non-enzymatic conditions by the amino groups of biomacromolecules and the free carbonyl groups of glucose or other reducing sugars commonly produced by thermally processed foods. AGEs can cause various diseases, such as diabetes, atherosclerosis, neurodegeneration, and chronic kidney disease, by triggering the receptors of AGE (RAGEs) in the human body. There is evidence that AGEs can also affect the different structures and physiological functions of the skin. However, the mechanism is complicated and cumbersome and causes various harms to the skin. This article aims to identify and summarise the formation and characteristics of AGEs, focussing on the molecular mechanisms by which AGEs affect the composition and structure of normal skin substances at different skin layers and induce skin issues. We also discuss prevention and inhibition pathways, provide a systematic and comprehensive method for measuring the content of AGEs in human skin, and summarise and analyse their advantages and disadvantages. This work can help researchers acquire a deeper understanding of the relationship between AGEs and the skin and provides a basis for the development of effective ingredients that inhibit glycation.
Keywords: advanced glycation end products; fibroblasts; keratinocytes; matrix metalloproteinase; measurement methods; protein cross-linking; skin barrier.
Copyright © 2022 Chen, Zhang, Li, Guo, He, Dong, Meng and Yi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Maillard LC. Action des acides amines sur les sucres; formation des melanoidines par voie methodique. Comptes R Acad Sci (Paris). (1912) 154:66–8.
-
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. (2001) 44:129–46. - PubMed
-
- Rasool M, Malik A, Butt TT, Ashraf MAB, Rasool R, Zahid A, et al. Implications of advanced oxidation protein products (AOPPs), advanced glycation end products (AGEs) and other biomarkers in the development of cardiovascular diseases. Saudi J Biol Sci. (2019) 26:334–9. 10.1016/j.sjbs.2018.08.024 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

