METORY: Development of a Demand-Driven Blockchain-Based Dynamic Consent Platform Tailored for Clinical Trials
- PMID: 35646983
- PMCID: PMC9136007
- DOI: 10.3389/fmed.2022.837197
METORY: Development of a Demand-Driven Blockchain-Based Dynamic Consent Platform Tailored for Clinical Trials
Abstract
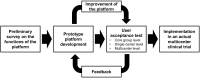
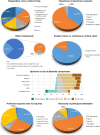
The recent advent of the dynamic consent concept intensified the data integrity issue in clinical trials. Incorporating blockchain technology into a dynamic consent platform can be a feasible solution. Due to various clinical trial settings, a demand-driven development strategy is required. We developed a blockchain-based dynamic consent platform named METORY tailored for clinical trials. The platform consisted of three parts: web and mobile application user interface, study management platform, and blockchain platform. Hyperledger Fabric, an enterprise-grade private blockchain framework, was used to integrate blockchain into the study consent platform. We conducted user acceptance tests and applied feedback to the improvement of the platform. Identity and role-based access control was constructed by combining mobile-application-based certificate system and access control functionalities in Hyperledger fabric. Data were encrypted using SHA-256 prior to transmission to blockchain server and TLS protocol was used for in-transit encryption. File-system level encryption was separated implemented within the security measures from Amazon RDS. Users' experience in the clinical trial was acceptable in the ease and usefulness of the platform.
Keywords: blockchain; clinical trials; dynamic consent; mobile application; platform.
Copyright © 2022 Huh, Jeong, Moon, Kim, Yang, Jeong, Song, Kwak, Lee and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Waterhouse DM, Harvey RD, Hurley P, Levit LA, Kim ES, Klepin HD, et al. . Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: findings from an American Society of Clinical Oncology survey. JCO Oncol Pract. (2020) 16:417–21. 10.1200/op.20.00275 - DOI - PubMed
LinkOut - more resources
Full Text Sources

