Optical Coherence Tomography: An Eye Into the Coronary Artery
- PMID: 35647059
- PMCID: PMC9130606
- DOI: 10.3389/fcvm.2022.854554
Optical Coherence Tomography: An Eye Into the Coronary Artery
Abstract
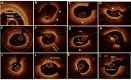
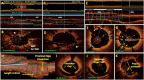
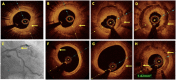
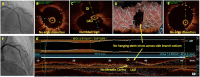
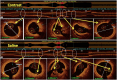
Optical coherence tomography (OCT) is slowly but surely gaining a foothold in the hands of interventional cardiologists. Intraluminal and transmural contents of the coronary arteries are no longer elusive to the cardiologist's probing eye. Although the graduation of an interventionalist in imaging techniques right from naked eye angiographies to ultrasound-based coronary sonographies to the modern light-based OCT has been slow, with the increasing regularity of complex coronary cases in practice, such a transition is inevitable. Although intravascular ultrasound (IVUS) due to its robust clinical data has been the preferred imaging modality in recent years, OCT provides a distinct upgrade over it in many imaging and procedural aspects. Better image resolution, accurate estimation of the calcified lesion, and better evaluation of acute and chronic stent failure are the distinct advantages of OCT over IVUS. Despite the obvious imaging advantages of OCT, its clinical impact remains subdued. However, upcoming newer trials and data have been encouraging for expanding the use of OCT to wider indications in clinical utility. During percutaneous coronary intervention (PCI), OCT provides the detailed information (dissection, tissue prolapse, thrombi, and incomplete stent apposition) required for optimal stent deployment, which is the key to successfully reducing the major adverse cardiovascular event (MACE) and stent-related morbidities. The increasing use of OCT in complex bifurcation stenting involving the left main (LM) is being studied. Also, the traditional pitfalls of OCT, such as additional contrast load for image acquisition and stenting involving the ostial and proximal LM, have also been overcome recently. In this review, we discuss the interpretation of OCT images and its clinical impact on the outcome of procedures along with current barriers to its use and newer paradigms in which OCT is starting to become a promising tool for the interventionalist and what can be expected for the immediate future in the imaging world.
Keywords: IVUS; OCT; OCT in ACS; OCT in bifurcation angioplasty; OCT in left main bifurcation angioplasty; calcified lesion modification; plaque morphology; saline OCT.
Copyright © 2022 Gupta, Shrivastava, Vijayvergiya, Chhikara, Datta, Aziz, Singh Meena, Nath and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

