The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders
- PMID: 35647072
- PMCID: PMC9136230
- DOI: 10.3389/fcvm.2022.897087
The Endothelial Glycocalyx: A Possible Therapeutic Target in Cardiovascular Disorders
Abstract
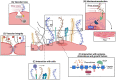
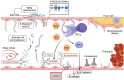
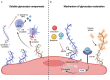
The physiological, anti-inflammatory, and anti-coagulant properties of endothelial cells (ECs) rely on a complex carbohydrate-rich layer covering the luminal surface of ECs, called the glycocalyx. In a range of cardiovascular disorders, glycocalyx shedding causes endothelial dysfunction and inflammation, underscoring the importance of glycocalyx preservation to avoid disease initiation and progression. In this review we discuss the physiological functions of the glycocalyx with particular focus on how loss of endothelial glycocalyx integrity is linked to cardiovascular risk factors, like hypertension, aging, diabetes and obesity, and contributes to the development of thrombo-inflammatory conditions. Finally, we consider the role of glycocalyx components in regulating inflammatory responses and discuss possible therapeutic interventions aiming at preserving or restoring the endothelial glycocalyx and therefore protecting against cardiovascular disease.
Keywords: atherosclerosis; cardiovascular risk factor; endothelial cell (EC); glycocalyx; heparan sulfate (HS); inflammation; ischemia/reperfusion injury; therapeutic target.
Copyright © 2022 Milusev, Rieben and Sorvillo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures