Global hemostatic profiling in patients with decompensated cirrhosis and bacterial infections
- PMID: 35647501
- PMCID: PMC9131254
- DOI: 10.1016/j.jhepr.2022.100493
Global hemostatic profiling in patients with decompensated cirrhosis and bacterial infections
Abstract
Background & aims: Bacterial infections in cirrhosis are associated with increased bleeding risk. To assess the factors responsible for bleeding tendency in patients with bacterial infections, we conducted a prospective study comparing all 3 aspects of hemostasis (platelets, coagulation, and fibrinolysis) in hospitalized patients with decompensated cirrhosis with vs. without bacterial infections.
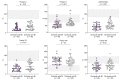
Methods: Primary hemostasis assessment included whole blood platelet aggregation and von Willebrand factor (VWF). Coagulation assessment included procoagulant factors (fibrinogen, factor II, V, VII, VIII, IX, X, XI, XII, XIII), natural anticoagulants (protein C, protein S, antithrombin) and thrombomodulin-modified thrombin generation test. Fibrinolysis assessment included fibrinolytic factors (plasminogen, t-PA, PAI-1, α2-AP, TAFIa/ai) and plasmin-antiplasmin complex (PAP).
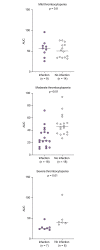
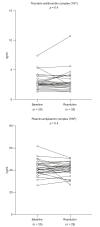
Results: Eighty patients with decompensated cirrhosis were included (40 with and 40 without bacterial infections). Severity of cirrhosis and platelet count were comparable between groups. At baseline, patients with cirrhosis and bacterial infections had significantly lower whole blood platelet aggregation, without significant differences in VWF. Regarding coagulation, bacterial infections were associated with reduced procoagulant factors VII and XII, and a significant reduction of all natural anticoagulants. However, thrombomodulin-modified thrombin generation was comparable between the study groups. Finally, although mixed potentially hypo-fibrinolytic (lower plasminogen) and hyper-fibrinolytic (higher t-PA) changes were present in bacterial infections, a comparable level of PAP was detected in both groups. Upon resolution of infection (n = 29/40), platelet aggregation further deteriorated whereas coagulation and fibrinolysis factors returned to levels observed in patients without bacterial infections.
Conclusion: In hospitalized patients with decompensated cirrhosis, bacterial infections are associated with reduced whole blood platelet aggregation and a significant decrease of all natural anticoagulants, which may unbalance hemostasis and potentially increase the risk of both bleeding and thrombosis.
Lay summary: Bacterial infections are a common issue in hospitalized patients with decompensated cirrhosis (i.e. patients hospitalized due to severe complications of advanced chronic liver disease). Patients with decompensated cirrhosis who acquire infections may be at increased risk of bleeding complications following invasive procedures (that is a procedure in which the body is penetrated or entered, for instance by a needle or a tube). As bleeding complications in decompensated cirrhosis are associated with a high risk of further decompensation and death, there is an urgent need to understand the factors responsible for such increased bleeding tendency. Herein, we investigated the alterations of hemostasis (that is the physiological process responsible for clot formation and stability) in patients with decompensated cirrhosis and bacterial infections. We found that development of bacterial infections in these patients is associated with alterations of hemostasis (particularly of platelets and clotting cascade) that may increase the risk of both bleeding and thrombotic complications.
Keywords: ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; AT, antithrombin; ETP, endogenous thrombin potential; F, factor; FXIII, fibrin-stabilizing factor XIII; MELD, model for end-stage liver disease; PAI-1, plasminogen activator inhibitor-1; PAP, plasmin-antiplasmin complex; PC, protein C; PS, protein S; TAFIa/ai, activated and inactivated thrombin-activatable fibrinolytic inhibitor; TM, thrombomodulin; VWF, von Willebrand factor; cirrhosis; coagulation; fibrinolysis; infections; platelets; t-PA, tissue-type plasminogen activator; α2-AP, α2-antiplasmin.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Zermatten M.G., Fraga M., Moradpour D., Bertaggia Calderara D., Aliotta A., Stirnimann G., et al. Hemostatic alterations in patients with cirrhosis: from primary hemostasis to fibrinolysis. Hepatology. 2020;71:2135–2148. - PubMed
-
- Lisman T., Intagliata N.M. Bleeding and thrombosis in patients with liver diseases. Semin Thromb Hemost. 2020;46:653–655. - PubMed
-
- Zanetto A., Senzolo M., Vitale A., Cillo U., Radu C., Sartorello F., et al. Thromboelastometry hypercoagulable profiles and portal vein thrombosis in cirrhotic patients with hepatocellular carcinoma. Dig Liver Dis. 2017;49:440–445. - PubMed
-
- Northup P.G., Garcia-Pagan J.C., Garcia-Tsao G., Intagliata N.M., Superina R.A., Roberts L.N., et al. Vascular liver disorders, portal vein thrombosis, and procedural bleeding in patients with liver disease: 2020 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;73:366–413. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

