Valuable effect of Manuka Honey in increasing the printability and chondrogenic potential of a naturally derived bioink
- PMID: 35647514
- PMCID: PMC9130107
- DOI: 10.1016/j.mtbio.2022.100287
Valuable effect of Manuka Honey in increasing the printability and chondrogenic potential of a naturally derived bioink
Abstract
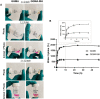
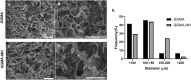
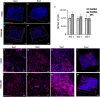
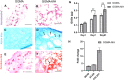
Hydrogel-based bioinks are the main formulations used for Articular Cartilage (AC) regeneration due to their similarity to chondral tissue in terms of morphological and mechanical properties. However, the main challenge is to design and formulate bioinks able to allow reproducible additive manufacturing and fulfil the biological needs for the required tissue. In our work, we investigated an innovative Manuka honey (MH)-loaded photocurable gellan gum methacrylated (GGMA) bioink, encapsulating mesenchymal stem cells differentiated in chondrocytes (MSCs-C), to generate 3D bioprinted construct for AC studies. We demonstrated the beneficial effect of MH incorporation on the bioink printability, leading to the obtainment of a more homogenous filament extrusion and therefore a better printing resolution. Also, GGMA-MH formulation showed higher viscoelastic properties, presenting complex modulus G∗ values of ∼1042 Pa, compared to ∼730 Pa of GGMA. Finally, MH-enriched bioink induced a higher expression of chondrogenic markers col2a1 (14-fold), sox9 (3-fold) and acan (4-fold) and AC ECM main element production (proteoglycans and collagen).
Keywords: Articular cartilage; Extrusion bioprinting; Manuka honey; Mesenchymal stem cells; Methacrylated gellan gum.
© 2022 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Annachiara Scalzone reports financial support was provided by 10.13039/501100000266Engineering and Physical Sciences Research Council. Kenny Dalgarno, Xiao Nong Wang reports financial support was provided by 10.13039/501100012041Versus Arthritis.
Figures




References
-
- Hu J.C.Y., Athanasiou K.A. In: Structure and Function of Articular Cartilage BT - Handbook of Histology Methods for Bone and Cartilage. An Y.H., Martin K.L., editors. Humana Press; Totowa, NJ: 2003. pp. 73–95.
-
- Mouser V.H.M., Levato R., Mensinga A., Wouter J.A., Gawlitta D., Malda J., et al. Bio-ink development for three-dimensional bioprinting of hetero-cellular cartilage constructs. Connect. Tissue Res. 2020;61(2):137–151. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

