Synthesis of a fluorinated pyronin that enables blue light to rapidly depolarize mitochondria
- PMID: 35647549
- PMCID: PMC9020612
- DOI: 10.1039/d1md00395j
Synthesis of a fluorinated pyronin that enables blue light to rapidly depolarize mitochondria
Abstract
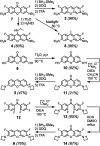
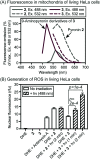
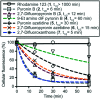
Fluorinated analogues of the fluorophore pyronin B were synthesized as a new class of amine-reactive drug-like small molecules. In water, 2,7-difluoropyronin B was found to reversibly react with primary amines to form covalent adducts. When this fluorinated analogue is added to proteins, these adducts undergo additional oxidation to yield fluorescent 9-aminopyronins. Irradiation with visible blue light enhances this oxidation step, providing a photochemical method to modify the biological properties of reactive amines. In living HeLa cells, 2,7-difluoropyronin B becomes localized in mitochondria, where it is partially transformed into fluorescent aminopyronins, as detected by spectral profiling confocal microscopy. Further excitation of these cells with the blue laser of a confocal microscope can depolarize mitochondria within seconds. This biological activity was only observed with 2,7-difluoropyronin B and was not detected with analogues such as pyronin B or 9-methyl-2,7-difluoropyronin B. This irradiation with blue light enhances the cellular production of reactive oxygen species (ROS), suggesting that increased ROS in mitochondria promotes the formation of aminopyronins that inactivate biomolecules critical for maintenance of mitochondrial membrane potential. The unique reactivity of 2,7-difluoropyronin B offers a novel tool for photochemical control of mitochondrial biology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






Similar articles
-
Mitochondrial reactive oxygen species generation and calcium increase induced by visible light in astrocytes.Ann N Y Acad Sci. 2004 Apr;1011:45-56. doi: 10.1007/978-3-662-41088-2_5. Ann N Y Acad Sci. 2004. PMID: 15126282
-
A faster, high resolution, mtPA-GFP-based mitochondrial fusion assay acquiring kinetic data of multiple cells in parallel using confocal microscopy.J Vis Exp. 2012 Jul 20;(65):e3991. doi: 10.3791/3991. J Vis Exp. 2012. PMID: 22847388 Free PMC article.
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy.Biochim Biophys Acta. 1998 Aug 10;1366(1-2):177-96. doi: 10.1016/s0005-2728(98)00112-1. Biochim Biophys Acta. 1998. PMID: 9714796 Review.
-
Considerations on the mechanism of action of artemisinin antimalarials: part 1--the 'carbon radical' and 'heme' hypotheses.Infect Disord Drug Targets. 2013 Aug;13(4):217-77. doi: 10.2174/1871526513666131129155708. Infect Disord Drug Targets. 2013. PMID: 24304352 Review.
Cited by
-
Improving the Brightness of Pyronin Fluorophore Systems through Quantum-Mechanical Predictions.J Phys Chem Lett. 2022 Sep 8;13(35):8312-8318. doi: 10.1021/acs.jpclett.2c02287. Epub 2022 Aug 30. J Phys Chem Lett. 2022. PMID: 36040023 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

