RpoN-Based stapled peptides with improved DNA binding suppress Pseudomonas aeruginosa virulence
- PMID: 35647551
- PMCID: PMC9020619
- DOI: 10.1039/d1md00371b
RpoN-Based stapled peptides with improved DNA binding suppress Pseudomonas aeruginosa virulence
Abstract
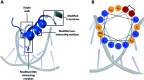
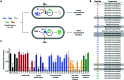
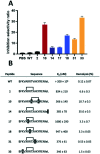
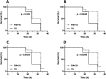
Stapled peptides have the ability to mimic α-helices involved in protein binding and have proved to be effective pharmacological agents for disrupting protein-protein interactions. DNA-binding proteins such as transcription factors bind their cognate DNA sequences via an α-helix interacting with the major groove of DNA. We previously developed a stapled peptide based on the bacterial alternative sigma factor RpoN capable of binding the RpoN DNA promoter sequence and inhibiting RpoN-mediated expression in Escherichia coli. We have elucidated a structure-activity relationship for DNA binding by this stapled peptide, improving DNA binding affinity constants in the high nM range. Lead peptides were shown to have low toxicity as determined by their low hemolytic activity at 100 μM and were shown to have anti-virulence activity in a Galleria mellonella model of Pseudomonas aeruginosa infection. These findings support further preclinical development of stapled peptides as antivirulence agents targeting P. aeruginosa.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Inhibition of Bacterial Gene Transcription with an RpoN-Based Stapled Peptide.Cell Chem Biol. 2018 Sep 20;25(9):1059-1066.e4. doi: 10.1016/j.chembiol.2018.05.007. Epub 2018 Jun 7. Cell Chem Biol. 2018. PMID: 29887265 Free PMC article.
-
Targeting the alternative sigma factor RpoN to combat virulence in Pseudomonas aeruginosa.Sci Rep. 2017 Oct 3;7(1):12615. doi: 10.1038/s41598-017-12667-y. Sci Rep. 2017. PMID: 28974743 Free PMC article.
-
Double Stranded DNA Binding Stapled Peptides: An Emerging Tool for Transcriptional Regulation.Chembiochem. 2023 Dec 14;24(24):e202300594. doi: 10.1002/cbic.202300594. Epub 2023 Oct 16. Chembiochem. 2023. PMID: 37750576 Review.
-
Differential roles of the Pseudomonas aeruginosa PA14 rpoN gene in pathogenicity in plants, nematodes, insects, and mice.J Bacteriol. 2001 Dec;183(24):7126-34. doi: 10.1128/JB.183.24.7126-7134.2001. J Bacteriol. 2001. PMID: 11717271 Free PMC article.
-
The Regulatory Functions of σ54 Factor in Phytopathogenic Bacteria.Int J Mol Sci. 2021 Nov 24;22(23):12692. doi: 10.3390/ijms222312692. Int J Mol Sci. 2021. PMID: 34884502 Free PMC article. Review.
Cited by
-
Stapled Peptides as Direct Inhibitors of Nrf2-sMAF Transcription Factors.J Med Chem. 2023 May 11;66(9):6184-6192. doi: 10.1021/acs.jmedchem.2c02037. Epub 2023 Apr 25. J Med Chem. 2023. PMID: 37097833 Free PMC article.
References
LinkOut - more resources
Full Text Sources

