Genome Editing for Sustainable Agriculture in Africa
- PMID: 35647578
- PMCID: PMC9133388
- DOI: 10.3389/fgeed.2022.876697
Genome Editing for Sustainable Agriculture in Africa
Abstract
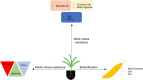
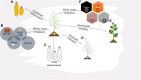
Sustainable intensification of agriculture in Africa is essential for accomplishing food and nutritional security and addressing the rising concerns of climate change. There is an urgent need to close the yield gap in staple crops and enhance food production to feed the growing population. In order to meet the increasing demand for food, more efficient approaches to produce food are needed. All the tools available in the toolbox, including modern biotechnology and traditional, need to be applied for crop improvement. The full potential of new breeding tools such as genome editing needs to be exploited in addition to conventional technologies. Clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas)-based genome editing has rapidly become the most prevalent genetic engineering approach for developing improved crop varieties because of its simplicity, efficiency, specificity, and easy to use. Genome editing improves crop variety by modifying its endogenous genome free of any foreign gene. Hence, genome-edited crops with no foreign gene integration are not regulated as genetically modified organisms (GMOs) in several countries. Researchers are using CRISPR/Cas-based genome editing for improving African staple crops for biotic and abiotic stress resistance and improved nutritional quality. Many products, such as disease-resistant banana, maize resistant to lethal necrosis, and sorghum resistant to the parasitic plant Striga and enhanced quality, are under development for African farmers. There is a need for creating an enabling environment in Africa with science-based regulatory guidelines for the release and adoption of the products developed using CRISPR/Cas9-mediated genome editing. Some progress has been made in this regard. Nigeria and Kenya have recently published the national biosafety guidelines for the regulation of gene editing. This article summarizes recent advances in developments of tools, potential applications of genome editing for improving staple crops, and regulatory policies in Africa.
Keywords: African crops; CRISPR/Cas; agriculture; genome editing; regulatory policies.
Copyright © 2022 Tripathi, Dhugga, Ntui, Runo, Syombua, Muiruri, Wen and Tripathi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Evolution and Application of Genome Editing Techniques for Achieving Food and Nutritional Security.Int J Mol Sci. 2021 May 25;22(11):5585. doi: 10.3390/ijms22115585. Int J Mol Sci. 2021. PMID: 34070430 Free PMC article. Review.
-
Genome Editing in Cereals: Approaches, Applications and Challenges.Int J Mol Sci. 2020 Jun 5;21(11):4040. doi: 10.3390/ijms21114040. Int J Mol Sci. 2020. PMID: 32516948 Free PMC article. Review.
-
CRISPR/Cas9: a sustainable technology to enhance climate resilience in major Staple Crops.Front Genome Ed. 2025 Mar 18;7:1533197. doi: 10.3389/fgeed.2025.1533197. eCollection 2025. Front Genome Ed. 2025. PMID: 40171546 Free PMC article. Review.
-
Recent advances of CRISPR-based genome editing for enhancing staple crops.Front Plant Sci. 2024 Sep 23;15:1478398. doi: 10.3389/fpls.2024.1478398. eCollection 2024. Front Plant Sci. 2024. PMID: 39376239 Free PMC article. Review.
-
Nanotechnology and CRISPR/Cas9 system for sustainable agriculture.Environ Sci Pollut Res Int. 2023 Dec;30(56):118049-118064. doi: 10.1007/s11356-023-26482-8. Epub 2023 Mar 27. Environ Sci Pollut Res Int. 2023. PMID: 36973619 Review.
Cited by
-
The Adoption of Genetically Modified Crops in Africa: the Public's Current Perception, the Regulatory Obstacles, and Ethical Challenges.GM Crops Food. 2024 Dec 31;15(1):1-15. doi: 10.1080/21645698.2024.2345401. Epub 2024 Apr 23. GM Crops Food. 2024. PMID: 38651587 Free PMC article. Review.
-
The African continent should consider a harmonized consultative and collaborative effort towards coordinated policy and regulatory guidelines across the fields of biotechnology.Front Bioeng Biotechnol. 2023 Jun 7;11:1211789. doi: 10.3389/fbioe.2023.1211789. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37351467 Free PMC article.
-
Africa and zero hunger agenda: genome editing policy landscape, challenges and opportunities.Front Bioeng Biotechnol. 2025 Mar 12;13:1526851. doi: 10.3389/fbioe.2025.1526851. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40144392 Free PMC article. Review.
-
Genome Editing and Improvement of Abiotic Stress Tolerance in Crop Plants.Life (Basel). 2023 Jun 27;13(7):1456. doi: 10.3390/life13071456. Life (Basel). 2023. PMID: 37511831 Free PMC article. Review.
-
Navigating biosafety regulatory frameworks for genetic engineering in Africa: a focus on genome editing and gene drive technologies.Front Bioeng Biotechnol. 2024 Oct 24;12:1483279. doi: 10.3389/fbioe.2024.1483279. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39512657 Free PMC article.
References
-
- Aboubacar A., Axtell J. D., Huang C.-P., Hamaker B. R. (2001). A Rapid Protein Digestibility Assay for Identifying Highly Digestible Sorghum Lines. Cereal Chem. J. 78, 160–165. 10.1094/cchem.2001.78.2.160 - DOI
-
- Amusa N. A., Adigbite A. A., Muhammed S., Baiyewu R. A. (2003). Yam Diseases and its Management in Nigeria. Afri. J. Biotechnol. 2 (12), 497–502. 10.5897/AJB2003.000-1099 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

