Assessment of State and Federal Health Policies for Opioid Use Disorder Treatment During the COVID-19 Pandemic and Beyond
- PMID: 35647581
- PMCID: PMC9138185
- DOI: 10.1001/jamahealthforum.2021.3833
Assessment of State and Federal Health Policies for Opioid Use Disorder Treatment During the COVID-19 Pandemic and Beyond
Erratum in
-
Errors in Figure 3.JAMA Health Forum. 2022 Jan 4;3(1):e214958. doi: 10.1001/jamahealthforum.2021.4958. JAMA Health Forum. 2022. PMID: 36218858 Free PMC article. No abstract available.
Abstract
Importance: Federal and state governments implemented temporary strategies for providing access to opioid use disorder (OUD) treatment during the COVID-19 pandemic. Advocates hope many of these policies become permanent because of their potential to expand access to care.
Objective: To consider the multitude of ways access to and utilization of treatment for individuals with OUD might have been expanded by state and federal policy so researchers can do a better job evaluating the effectiveness of specific policy approaches, which will depend on the interaction with other state policies.
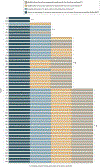
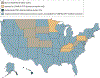
Evidence review: We summarize state-level policy data reported by government and nonprofit agencies that track health care regulations, specifically the Kaiser Family Foundation, Federation of State Medical Boards, American Association of Nurse Practitioners, American Academy of Physician Assistants, and the National Safety Council. Data were collected by these sources from September 2020 through January 2021. We examine heterogeneity in policy elements adopted across states during the COVID-19 pandemic in 4 key areas: telehealth, privacy, licensing, and medication for opioid use disorder. The analysis was conducted from March 2020 through January 2021.
Findings: This cross-sectional study found that federal and state governments have taken important steps to ensure OUD treatment availability during the COVID-19 pandemic, but few states are comprehensive in their approach. Although all states and Washington, DC have adopted at least 1 telehealth policy, only 17 states have adopted telehealth policies that improve access to OUD treatment for new patients. Furthermore, only 9 states relaxed privacy laws, which influence the ability to use particular technology for telehealth visits. Similarly, all states have adopted at least 1 policy related to health care professional licensing permissions, but only 35 expanded the scope of practice laws for both physician assistants and nurse practitioners. Forty-four states expanded access to initiation and delivery of medication for OUD treatment. Together, no state has implemented all of these policies to comprehensively expand access to OUD treatment during the COVID-19 pandemic.
Conclusions and relevance: With considerable policy changes potentially affecting access to treatment and treatment retention for patients with OUD during the pandemic, evaluations must account for the variation in state approaches in related policy areas because the interactions between policies may limit the potential effectiveness of any single policy approach.
Figures

Similar articles
-
Synthesising evidence of the effects of COVID-19 regulatory changes on methadone treatment for opioid use disorder: implications for policy.Lancet Public Health. 2023 Mar;8(3):e238-e246. doi: 10.1016/S2468-2667(23)00023-3. Lancet Public Health. 2023. PMID: 36841564 Free PMC article. Review.
-
Receipt of Telehealth Services, Receipt and Retention of Medications for Opioid Use Disorder, and Medically Treated Overdose Among Medicare Beneficiaries Before and During the COVID-19 Pandemic.JAMA Psychiatry. 2022 Oct 1;79(10):981-992. doi: 10.1001/jamapsychiatry.2022.2284. JAMA Psychiatry. 2022. PMID: 36044198 Free PMC article.
-
The estimated impact of state-level support for expanded delivery of substance use disorder treatment during the COVID-19 pandemic.Addiction. 2022 Jun;117(6):1781-1786. doi: 10.1111/add.15778. Epub 2021 Dec 27. Addiction. 2022. PMID: 34873783 Free PMC article.
-
Use of and Retention on Video, Telephone, and In-Person Buprenorphine Treatment for Opioid Use Disorder During the COVID-19 Pandemic.JAMA Netw Open. 2022 Oct 3;5(10):e2236298. doi: 10.1001/jamanetworkopen.2022.36298. JAMA Netw Open. 2022. PMID: 36223118 Free PMC article.
-
Utilization of Telehealth Solutions for Patients with Opioid Use Disorder Using Buprenorphine: A Scoping Review.Telemed J E Health. 2022 Jun;28(6):761-767. doi: 10.1089/tmj.2021.0308. Epub 2021 Oct 29. Telemed J E Health. 2022. PMID: 34714172
Cited by
-
Telemedicine Buprenorphine Initiation and Retention in Opioid Use Disorder Treatment for Medicaid Enrollees.JAMA Netw Open. 2023 Oct 2;6(10):e2336914. doi: 10.1001/jamanetworkopen.2023.36914. JAMA Netw Open. 2023. PMID: 37851446 Free PMC article.
-
Expert Panel Consensus on State-Level Policies to Improve Engagement and Retention in Treatment for Opioid Use Disorder.JAMA Health Forum. 2022 Sep 2;3(9):e223285. doi: 10.1001/jamahealthforum.2022.3285. JAMA Health Forum. 2022. PMID: 36218944 Free PMC article.
-
Synthesising evidence of the effects of COVID-19 regulatory changes on methadone treatment for opioid use disorder: implications for policy.Lancet Public Health. 2023 Mar;8(3):e238-e246. doi: 10.1016/S2468-2667(23)00023-3. Lancet Public Health. 2023. PMID: 36841564 Free PMC article. Review.
-
Estimating the impact of the COVID-19 pandemic on rising trends in drug overdose mortality in the United States, 2018-2021.Ann Epidemiol. 2023 Jan;77:85-89. doi: 10.1016/j.annepidem.2022.11.007. Epub 2022 Nov 28. Ann Epidemiol. 2023. PMID: 36455852 Free PMC article.
-
An overview and visual analysis of research on government regulation in healthcare.Front Public Health. 2023 Nov 13;11:1272572. doi: 10.3389/fpubh.2023.1272572. eCollection 2023. Front Public Health. 2023. PMID: 38026398 Free PMC article. Review.
References
-
- Provisional Drug Overdose Death Counts. 2021. Accessed September 26, 2021. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
-
- Robinson E, Daly M. Explaining the rise and fall of psychological distress during the COVID-19 crisis in the United States: longitudinal evidence from the Understanding America Study. Br J Health Psychol. 2021;26(2):570–587. - PubMed
-
- Stephenson J. CDC warns of surge in drug overdose deaths during COVID-19. JAMA Health Forum. 2021;2:e210001–e. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

