Inhibition of Staphylococcus aureus Biofilm Formation and Virulence Factor Production by Petroselinic Acid and Other Unsaturated C18 Fatty Acids
- PMID: 35647620
- PMCID: PMC9241682
- DOI: 10.1128/spectrum.01330-22
Inhibition of Staphylococcus aureus Biofilm Formation and Virulence Factor Production by Petroselinic Acid and Other Unsaturated C18 Fatty Acids
Abstract
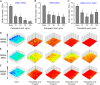
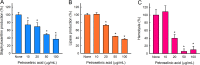
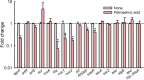
Staphylococcus aureus is a major human pathogen that secretes several toxins associated with the pathogenesis of sepsis and pneumonia. Its antibiotic resistance is notorious, and its biofilms play a critical role in antibiotic tolerance. We hypothesized fatty acids might inhibit S. aureus biofilm formation and the expressions of its virulence factors. Initially, the antibiofilm activities of 27 fatty acids against a methicillin-sensitive S. aureus strain were investigated. Of the fatty acids tested, three C18 unsaturated fatty acids, that is, petroselinic, vaccenic, and oleic acids at 100 μg/mL, inhibited S. aureus biofilm formation by more than 65% without affecting its planktonic cell growth (MICs were all > 400 μg/mL). Notably, petroselinic acid significantly inhibited biofilm formation of two methicillin-resistant S. aureus strains and two methicillin-sensitive S. aureus strains. In addition, petroselinic acid significantly suppressed the production of three virulence factors, namely, staphyloxanthin, lipase, and α-hemolysin. Transcriptional analysis showed that petroselinic acid repressed the gene expressions of quorum sensing regulator agrA, effector of quorum sensing RNAIII, α-hemolysin hla, nucleases nuc1 and nuc2, and the virulence regulator saeR. Furthermore, petroselinic acid dose-dependently inhibited S. aureus biofilm formation on abiotic surfaces and porcine skin. These findings suggest that fatty acids, particularly petroselinic acid, are potentially useful for controlling biofilm formation by S. aureus. IMPORTANCE Fatty acids with a long carbon chain have recently attracted attention because of their antibiofilm activities against microbes. Here, we report the antibiofilm activities of 27 fatty acids against S. aureus. Of the fatty acids tested, three C18 unsaturated fatty acids (petroselinic, vaccenic, and oleic acids) significantly inhibited biofilm formation by S. aureus. Furthermore, petroselinic acid inhibited the production of several virulence factors in S. aureus. The study also reveals that the action mechanism of petroselinic acid involves repression of quorum-sensing-related and virulence regulator genes. These findings show that natural and nontoxic petroselinic acid has potential use as a treatment for S. aureus infections, including infections by methicillin-resistant S. aureus strains, and in food processing facilities.
Keywords: Staphylococcus aureus; biofilm; fatty acids; hemolysis; petroselinic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Hydroquinones Inhibit Biofilm Formation and Virulence Factor Production in Staphylococcus aureus.Int J Mol Sci. 2022 Sep 14;23(18):10683. doi: 10.3390/ijms231810683. Int J Mol Sci. 2022. PMID: 36142597 Free PMC article.
-
Quorum sensing mediated attenuation of biofilm formation and virulence traits in Staphylococcus aureus by trigonelline.Microb Pathog. 2025 Aug;205:107731. doi: 10.1016/j.micpath.2025.107731. Epub 2025 May 21. Microb Pathog. 2025. PMID: 40409430
-
The effectiveness of anti-biofilm and anti-virulence properties of dihydrocelastrol and dihydrocelastryl diacetate in fighting against methicillin-resistant Staphylococcus aureus.Arch Microbiol. 2017 Oct;199(8):1151-1163. doi: 10.1007/s00203-017-1386-x. Epub 2017 May 9. Arch Microbiol. 2017. PMID: 28487997
-
Disarming Staphylococcus aureus: Review of Strategies Combating This Resilient Pathogen by Targeting Its Virulence.Pathogens. 2025 Apr 15;14(4):386. doi: 10.3390/pathogens14040386. Pathogens. 2025. PMID: 40333163 Free PMC article. Review.
-
Methicillin resistance and the biofilm phenotype in Staphylococcus aureus.Front Cell Infect Microbiol. 2015 Jan 28;5:1. doi: 10.3389/fcimb.2015.00001. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25674541 Free PMC article. Review.
Cited by
-
Antibiofilm and Antihemolytic Activities of Actinostemma lobatum Extract Rich in Quercetin against Staphylococcus aureus.Pharmaceutics. 2024 Aug 16;16(8):1075. doi: 10.3390/pharmaceutics16081075. Pharmaceutics. 2024. PMID: 39204420 Free PMC article.
-
Antibacterial and antibiofilm activities of iodinated hydrocarbons against Vibrio parahaemolyticus and Staphylococcus aureus.Sci Rep. 2024 Apr 22;14(1):9160. doi: 10.1038/s41598-024-55479-7. Sci Rep. 2024. PMID: 38644387 Free PMC article.
-
Watercress oil loaded with gel: evaluation of hemolysis inhibition, antioxidant, antimicrobial, and healing properties.Front Pharmacol. 2024 Aug 23;15:1424369. doi: 10.3389/fphar.2024.1424369. eCollection 2024. Front Pharmacol. 2024. PMID: 39246652 Free PMC article.
-
Optimizing lipase production by Bacillus subtilis on cheese whey and evaluating its antimicrobial, antibiofilm, anti virulence and biosafety properties.Sci Rep. 2025 Apr 1;15(1):11087. doi: 10.1038/s41598-025-92181-8. Sci Rep. 2025. PMID: 40169631 Free PMC article.
-
Inhibition of Biofilm Formation in Cutibacterium acnes, Staphylococcus aureus, and Candida albicans by the Phytopigment Shikonin.Int J Mol Sci. 2024 Feb 19;25(4):2426. doi: 10.3390/ijms25042426. Int J Mol Sci. 2024. PMID: 38397101 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

