Population Pharmacokinetics of Remdesivir and GS-441524 in Hospitalized COVID-19 Patients
- PMID: 35647646
- PMCID: PMC9211420
- DOI: 10.1128/aac.00254-22
Population Pharmacokinetics of Remdesivir and GS-441524 in Hospitalized COVID-19 Patients
Abstract
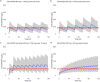
The objective of this study was to describe the population pharmacokinetics of remdesivir and GS-441524 in hospitalized coronavirus disease 2019 (COVID-19) patients. A prospective observational pharmacokinetic study was performed in non-critically ill hospitalized COVID-19 patients with hypoxemia. For evaluation of the plasma concentrations of remdesivir and its metabolite GS-441524, samples were collected on the first day of therapy. A nonlinear mixed-effects model was developed to describe the pharmacokinetics and identify potential covariates that explain variability. Alternative dosing regimens were evaluated using Monte Carlo simulations. Seventeen patients were included. Remdesivir and GS-441524 pharmacokinetics were best described by a one-compartment model. The estimated glomerular filtration rate (eGFR) on GS-441524 clearance was identified as a clinically relevant covariate. The interindividual variability in clearance and volume of distribution for both remdesivir and GS-441524 was high (remdesivir, 38.9% and 47.9%, respectively; GS-441525, 47.4% and 42.9%, respectively). The estimated elimination half-life for remdesivir was 0.48 h, and that for GS-441524 was 26.6 h. The probability of target attainment (PTA) of the in vitro 50% effective concentration (EC50) for GS-441524 in plasma can be improved by shortening the dose interval of remdesivir and thereby increasing the total daily dose (PTA, 51.4% versus 94.7%). In patients with reduced renal function, the metabolite GS-441524 accumulates. A population pharmacokinetic model for remdesivir and GS-441524 in COVID-19 patients was developed. Remdesivir showed highly variable pharmacokinetics. The elimination half-life of remdesivir in COVID-19 patients is short, and the clearance of GS-441524 is dependent on the eGFR. Alternative dosing regimens aimed at optimizing the remdesivir and GS-441524 concentrations may improve the effectiveness of remdesivir treatment in COVID-19 patients.
Keywords: COVID-19; pharmacokinetics; remdesivir.
Conflict of interest statement
The authors declare a conflict of interest. The Hague Hospital Pharmacy (E.L. and E.B.W.) received a research grant from Gilead Sciences Inc., unrelated to the submitted work. T.H.O. participates in a COVID-19 Digital Advisory Board from Gilead Sciences Inc. All other authors: none to declare.
Figures


References
-
- NIH. 2021. Therapeutic management of hospitalized adults with COVID-19. NIH, Bethesda, MD. https://www.covid19treatmentguidelines.nih.gov/management/clinical-manag....
-
- Qaseem A, Yost J, Etxeandia-Ikobaltzeta I, Abraham GM, Jokela JA, Forciea MA, Miller MC, Humphrey LL, Scientific Medical Policy Committee of the American College of Physicians. 2021. Update alert 2: should remdesivir be used for the treatment of patients with COVID-19? Rapid, living practice points from the American College of Physicians (version 2). Ann Intern Med 174:W116–W117. 10.7326/L21-0607. - DOI - PMC - PubMed
-
- Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, Diallo A, Lê M-P, Peytavin G, Staub T, Greil R, Guedj J, Paiva J-A, Costagliola D, Yazdanpanah Y, Burdet C, Mentré F, DisCoVeRy Study Group. 2022. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis 22:209–221. 10.1016/S1473-3099(21)00485-0. - DOI - PMC - PubMed
-
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M-D, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members. 2020. Remdesivir for the treatment of Covid-19—final report. N Engl J Med 383:1813–1826. 10.1056/NEJMoa2007764. - DOI - PMC - PubMed
-
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo A-M, Preziosi M-P, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny M-P, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, et al.. 2021. Repurposed antiviral drugs for COVID-19—interim WHO Solidarity Trial results. N Engl J Med 384:497–511. 10.1056/NEJMoa2023184. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

