Compartmentalized into Bacteriocytes but Highly Invasive: the Puzzling Case of the Co-Obligate Symbiont Serratia symbiotica in the Aphid Periphyllus lyropictus
- PMID: 35647657
- PMCID: PMC9241954
- DOI: 10.1128/spectrum.00457-22
Compartmentalized into Bacteriocytes but Highly Invasive: the Puzzling Case of the Co-Obligate Symbiont Serratia symbiotica in the Aphid Periphyllus lyropictus
Abstract
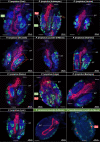
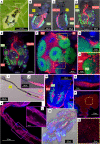
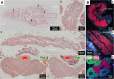
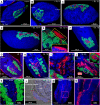
Dependence on multiple nutritional symbionts that form a metabolic unit has evolved many times in insects. Although it has been postulated that host dependence on these metabolically interconnected symbionts is sustained by their high degree of anatomical integration (these symbionts are often housed in distinct symbiotic cells, the bacteriocytes, assembled into a common symbiotic organ, the bacteriome), the developmental aspects of such multipartner systems have received little attention. Aphids of the subfamilies Chaitophorinae and Lachninae typically harbor disymbiotic systems in which the metabolic capabilities of the ancient obligate symbiont Buchnera aphidicola are complemented by those of a more recently acquired nutritional symbiont, often belonging to the species Serratia symbiotica. Here, we used microscopy approaches to finely characterize the tissue tropism and infection dynamics of the disymbiotic system formed by B. aphidicola and S. symbiotica in the Norway maple aphid Periphyllus lyropictus (Chaitophorinae). Our observations show that, in this aphid, the co-obligate symbiont S. symbiotica exhibits a dual lifestyle: intracellular by being housed in large syncytial bacteriocytes embedded between B. aphidicola-containing bacteriocytes in a well-organized compartmentalization pattern, and extracellular by massively invading the digestive tract and other tissues during embryogenesis. This is the first reported case of an obligate aphid symbiont that is internalized in bacteriocytes but simultaneously adopts an extracellular lifestyle. This unusual infection pattern for an obligate insect symbiont suggests that some bacteriocyte-associated obligate symbionts, despite their integration into a cooperative partnership, still exhibit invasive behavior and escape strict compartmentalization in bacteriocytes. IMPORTANCE Multipartner nutritional endosymbioses have evolved many times in insects. In Chaitophorinae aphids, the eroded metabolic capabilities of the ancient obligate symbiont B. aphidicola are complemented by those of more recently acquired symbionts. Here, we report the atypical case of the co-obligate S. symbiotica symbiont associated with P. lyropictus. This bacterium is compartmentalized into bacteriocytes nested into the ones harboring the more ancient symbiont B. aphidicola, reflecting metabolic convergences between the two symbionts. At the same time, S. symbiotica exhibits highly invasive behavior by colonizing various host tissues, including the digestive tract during embryogenesis. The discovery of this unusual phenotype for a co-obligate symbiont reveals a new face of multipartner nutritional endosymbiosis in insects. In particular, it shows that co-obligate symbionts can retain highly invasive traits and suggests that host dependence on these bacterial partners may evolve prior to their strict compartmentalization into specialized host structures.
Keywords: aphids; bacterial mutualism; bacteriocytes; co-obligate Serratia symbiotica; embryo invasion; gut symbiont.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mao M, Yang X, Poff K, Bennett G. 2017. Comparative genomics of the dual-obligate symbionts from the treehopper, Entylia carinata (Hemiptera: Membracidae), provide insight into the origins and evolution of an ancient symbiosis. Genome Biol Evol 9:1803–1815. doi: 10.1093/gbe/evx134. - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources

