Electron-Rich EDOT Linkers in Tetracationic bis-Triarylborane Chromophores: Influence on Water Stability, Biomacromolecule Sensing, and Photoinduced Cytotoxicity
- PMID: 35647673
- PMCID: PMC9543662
- DOI: 10.1002/chem.202201130
Electron-Rich EDOT Linkers in Tetracationic bis-Triarylborane Chromophores: Influence on Water Stability, Biomacromolecule Sensing, and Photoinduced Cytotoxicity
Abstract
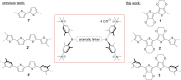
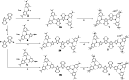
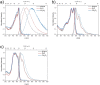
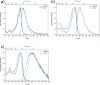
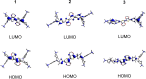
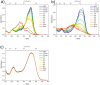
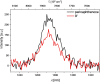
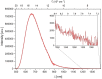
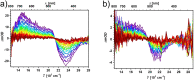
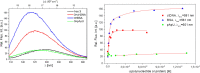
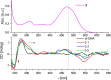
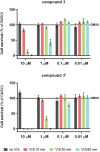
Three novel tetracationic bis-triarylboranes with 3,4-ethylenedioxythiophene (EDOT) linkers, and their neutral precursors, showed significant red-shifted absorption and emission compared to their thiophene-containing analogues, with one of the EDOT-derivatives emitting in the NIR region. Only the EDOT-linked trixylylborane tetracation was stable in aqueous solution, indicating that direct attachment of a thiophene or even 3-methylthiophene to the boron atom is insufficient to provide hydrolytic stability in aqueous solution. Further comparative analysis of the EDOT-linked trixylylborane tetracation and its bis-thiophene analogue revealed efficient photo-induced singlet oxygen production, with the consequent biological implications. Thus, both analogues bind strongly to ds-DNA and BSA, very efficiently enter living human cells, accumulate in several different cytoplasmic organelles with no toxic effect but, under intense visible light irradiation, they exhibit almost instantaneous and very strong cytotoxic effects, presumably attributed to singlet oxygen production. Thus, both compounds are intriguing theranostic agents, whose intracellular and probably intra-tissue location can be monitored by strong fluorescence, allowing switching on of the strong bioactivity by well-focused visible light.
Keywords: DNA/RNA sensors; boranes; fluorescent probes; singlet oxygen; theranostics.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Entwistle C. D., Marder T. B., Angew. Chem. Int. Ed. 2002, 41, 2927–2931; - PubMed
- Angew. Chem. 2002, 114, 3051–3056.
-
- Entwistle C. D., Marder T. B., Chem. Mater. 2004, 16, 4574–4585.
-
- Yamaguchi S., Wakamiya A., Pure Appl. Chem. 2006, 78, 1413–1424.
-
- Jäkle F., Coord. Chem. Rev. 2006, 250, 1107–1121.
-
- Elbing M., Bazan G. C., Angew. Chem. Int. Ed. 2008, 47, 834–838; - PubMed
- Angew. Chem. 2008, 120, 846–850.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

