A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor
- PMID: 35647711
- PMCID: PMC9234956
- DOI: 10.1021/acs.jmedchem.1c01856
A Small-Molecule Oral Agonist of the Human Glucagon-like Peptide-1 Receptor
Abstract
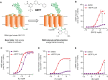
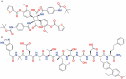
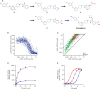
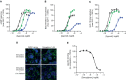
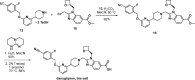
Peptide agonists of the glucagon-like peptide-1 receptor (GLP-1R) have revolutionized diabetes therapy, but their use has been limited because they require injection. Herein, we describe the discovery of the orally bioavailable, small-molecule, GLP-1R agonist PF-06882961 (danuglipron). A sensitized high-throughput screen was used to identify 5-fluoropyrimidine-based GLP-1R agonists that were optimized to promote endogenous GLP-1R signaling with nanomolar potency. Incorporation of a carboxylic acid moiety provided considerable GLP-1R potency gains with improved off-target pharmacology and reduced metabolic clearance, ultimately resulting in the identification of danuglipron. Danuglipron increased insulin levels in primates but not rodents, which was explained by receptor mutagensis studies and a cryogenic electron microscope structure that revealed a binding pocket requiring a primate-specific tryptophan 33 residue. Oral administration of danuglipron to healthy humans produced dose-proportional increases in systemic exposure (NCT03309241). This opens an opportunity for oral small-molecule therapies that target the well-validated GLP-1R for metabolic health.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.S.K., A.M.M., M.C.G., J.M.D., C.B., C.L., S.W.B., D.L., P.M.L., D.R.D., J.M.C., J.-P.F., Y.L., A.R.S., D.A.T., D.W.P., S.H., M.S.L., and D.A.G. are employees and stockholders of Pfizer Inc. J.B.K., D.J.E., R.B.R., D.A.P., and V.M.J. are stockholders of Pfizer Inc. J.C.P. is an employee and stockholder of Sosei Heptares.
Figures


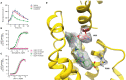




References
-
- Orskov C.; Holst J. J.; Knuhtsen S.; Baldissera F. G.; Poulsen S. S.; Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology 1986, 119, 1467–1475. 10.1210/endo-119-4-1467. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Molecular Biology Databases

