Patterns of treatment discontinuation in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration
- PMID: 35647983
- PMCID: PMC9359266
- DOI: 10.4103/ijo.IJO_3066_21
Patterns of treatment discontinuation in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration
Abstract
Purpose: To report the reasons for treatment discontinuation within 5 years in patients receiving intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy for neovascular age-related macular degeneration (nAMD).
Methods: A retrospective case-notes review of patients commenced on anti-VEGF for nAMD who failed to complete 5 years of follow-up was undertaken. The reasons for treatment discontinuation, baseline age, baseline visual acuity (VA) in Early Treatment Diabetic Retinopathy Study (ETDRS) letters, and the VA change at the last follow-up were recorded. Age-specific all-cause mortality was calculated for deceased patients.
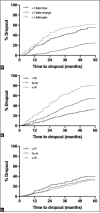
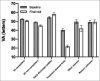
Results: Of the 1177 patients, 551 patients (46.8%) failed to complete the 5-year follow-up. The reasons for treatment discontinuation were death (251), early discharge due to stable disease (110), further treatment deemed futile (100), failure to attend (15), ill health (14), patient choice (7), and transfer of care (1). In 53 patients, no reason was documented. The mean baseline age of those who completed the 5-year follow-up (77.4 ± 7.8 years, 95% confidence interval (CI): 76.8-77.9) was significantly lower than those who discontinued the treatment for any reason (82 ± 7.7 years, 95% CI: 81.4-82.6) (P < 0.0001). Survival analysis showed that baseline VA was not a factor in treatment discontinuation; however, visual stability (±5 letters from baseline) was associated with treatment continuation. The age-specific all-cause mortality in deceased patients was lower than that in the general population.
Conclusion: At 5 years, only 53% of patients remained in active care, and death was the most common reason for treatment discontinuation. Lower baseline age and VA stability during therapy were associated with treatment continuation.
Keywords: Anti VEGF; nAMD; treatment discontinuation.
Conflict of interest statement
None
Figures
Comment in
-
Commentary: Patterns of treatment discontinuation in neovascular age-related macular degeneration.Indian J Ophthalmol. 2022 Jun;70(6):2070-2071. doi: 10.4103/ijo.IJO_825_22. Indian J Ophthalmol. 2022. PMID: 35647984 Free PMC article. No abstract available.
References
-
- GBD 2019 Blindness and Vision Impairment Collaborators;Vision Loss Expert Group of the Global Burden of Disease Study, Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020:The right to sight:An analysis for the Global Burden of disease study. Lancet Glob Health. 2021;9:e144–60a. - PMC - PubMed
-
- Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040:A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16. - PubMed
-
- Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration:Two-year results of the ANCHOR study. Ophthalmology. 2009;116:57–65.e5. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical