In vivo investigation of ruminant placenta function and physiology-a review
- PMID: 35648127
- PMCID: PMC9159061
- DOI: 10.1093/jas/skac045
In vivo investigation of ruminant placenta function and physiology-a review
Abstract
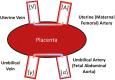
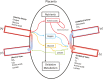
The placenta facilitates the transport of nutrients to the fetus, removal of waste products from the fetus, immune protection of the fetus and functions as an endocrine organ, thereby determining the environment for fetal growth and development. Additionally, the placenta is a highly metabolic organ in itself, utilizing a majority of the oxygen and glucose derived from maternal circulation. Consequently, optimal placental function is required for the offspring to reach its genetic potential in utero. Among ruminants, pregnant sheep have been used extensively for investigating pregnancy physiology, in part due to the ability to place indwelling catheters within both maternal and fetal vessels, allowing for steady-state investigation of blood flow, nutrient uptakes and utilization, and hormone secretion, under non-stressed and non-anesthetized conditions. This methodology has been applied to both normal and compromised pregnancies. As such, our understanding of the in vivo physiology of pregnancy in sheep is unrivalled by any other species. However, until recently, a significant deficit existed in determining the specific function or significance of individual genes expressed by the placenta in ruminants. To that end, we developed and have been using in vivo RNA interference (RNAi) within the sheep placenta to examine the function and relative importance of genes involved in conceptus development (PRR15 and LIN28), placental nutrient transport (SLC2A1 and SLC2A3), and placenta-derived hormones (CSH). A lentiviral vector is used to generate virus that is stably integrated into the infected cell's genome, thereby expressing a short-hairpin RNA (shRNA), that when processed within the cell, combines with the RNA Induced Silencing Complex (RISC) resulting in specific mRNA degradation or translational blockage. To accomplish in vivo RNAi, day 9 hatched and fully expanded blastocysts are infected with the lentivirus for 4 to 5 h, and then surgically transferred to synchronized recipient uteri. Only the trophectoderm cells are infected by the replication deficient virus, leaving the inner cell mass unaltered, and we often obtain ~70% pregnancy rates following transfer of a single blastocyst. In vivo RNAi coupled with steady-state study of blood flow and nutrient uptake, transfer and utilization can now provide new insight into the physiological consequences of modifying the translation of specific genes expressed within the ruminant placenta.
Keywords: Fick principle; RNA interference; blood flow; nutrients; placenta; ruminant.
Plain language summary
Optimal placental function is required for offspring to reach their genetic potential in utero, and functional placental insufficiency not only results in increased offspring morbidity and mortality, but can impact production traits long-term. However, assessing placental function in vivo is technically demanding, and robust assessment of placental function requires cannulating both maternal and fetal vasculature in order to obtain arterial and venous blood samples simultaneously under non-stressed/non-anesthetized conditions. While feasible in cattle, this approach has been used more extensively in sheep, providing a thorough understanding of placental nutrient uptake, transport, and utilization in normal and compromised pregnancies. Previously, it has not been feasible to alter the abundance of specific gene products within the ruminant placenta, impairing the direct assessment of “cause and effect” relationships in vivo. However, recently methods have been developed to facilitate RNA interference (RNAi) within the placenta, effectively generating a deficiency in specific gene products, to examine the impact on pregnancy progression and outcome. While in vivo RNAi is feasible in a variety of species, in sheep it is being coupled with the aforementioned approaches assessing in vivo placental function, thereby providing new insight into the ramification of specific gene function within ruminant placenta.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society of Animal Science. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



Similar articles
-
Impact of chorionic somatomammotropin RNA interference on uterine blood flow and placental glucose uptake in the absence of intrauterine growth restriction.Am J Physiol Regul Integr Comp Physiol. 2021 Feb 1;320(2):R138-R148. doi: 10.1152/ajpregu.00223.2020. Epub 2020 Nov 4. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 33146554 Free PMC article.
-
Assessing gene function in the ruminant placenta.Soc Reprod Fertil Suppl. 2010;67:119-31. Soc Reprod Fertil Suppl. 2010. PMID: 21755667
-
CSH RNA Interference Reduces Global Nutrient Uptake and Umbilical Blood Flow Resulting in Intrauterine Growth Restriction.Int J Mol Sci. 2021 Jul 29;22(15):8150. doi: 10.3390/ijms22158150. Int J Mol Sci. 2021. PMID: 34360913 Free PMC article.
-
The pregnant sheep as a model for human pregnancy.Theriogenology. 2008 Jan 1;69(1):55-67. doi: 10.1016/j.theriogenology.2007.09.021. Epub 2007 Nov 5. Theriogenology. 2008. PMID: 17976713 Free PMC article. Review.
-
Programming the offspring through altered uteroplacental hemodynamics: how maternal environment impacts uterine and umbilical blood flow in cattle, sheep and pigs.Reprod Fertil Dev. 2011;24(1):97-104. doi: 10.1071/RD11910. Reprod Fertil Dev. 2011. PMID: 22394721 Review.
Cited by
-
CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages.Animals (Basel). 2025 Mar 19;15(6):876. doi: 10.3390/ani15060876. Animals (Basel). 2025. PMID: 40150405 Free PMC article.
-
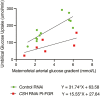
Increasing maternal glucose concentrations is insufficient to restore placental glucose transfer in chorionic somatomammotropin RNA interference pregnancies.Am J Physiol Endocrinol Metab. 2024 May 1;326(5):E602-E615. doi: 10.1152/ajpendo.00331.2023. Epub 2024 Feb 14. Am J Physiol Endocrinol Metab. 2024. PMID: 38353640 Free PMC article.
-
Impact of Chorionic Somatomammotropin In Vivo RNA Interference Phenotype on Uteroplacental Expression of the IGF Axis.Life (Basel). 2023 May 26;13(6):1261. doi: 10.3390/life13061261. Life (Basel). 2023. PMID: 37374044 Free PMC article.
-
Loci associated with spontaneous abortion in primiparous Holstein cattle.Front Vet Sci. 2025 May 30;12:1599401. doi: 10.3389/fvets.2025.1599401. eCollection 2025. Front Vet Sci. 2025. PMID: 40520427 Free PMC article.
-
W3112: Reproductive Performance in Domestic Ruminants.J Anim Sci. 2022 Jun 1;100(6):skac162. doi: 10.1093/jas/skac162. J Anim Sci. 2022. PMID: 35648130 Free PMC article. No abstract available.
References
-
- Alexander, G., and Williams D.. . 1971. Heat stress and development of the conceptus in domestic sheep. J. Agric. Sci. 76:53–72. doi:10.1017/S0021859600015616. - DOI
-
- Ali, A., Stenglein M. D., Spencer T. E., Bouma G. J., Anthony R. V., and Winger Q. A.. . 2020a. Trophectoderm-specific knockdown of Lin28 decreases expression of genes necessary for cell proliferation and reduces elongation of sheep conceptus. Int. J. Mol. Sci. 21:2549. doi:10.3390/ijms21072549. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

