Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: review and compilation of reactions
- PMID: 35648190
- PMCID: PMC9159052
- DOI: 10.1007/s00204-022-03304-3
Roles of selected non-P450 human oxidoreductase enzymes in protective and toxic effects of chemicals: review and compilation of reactions
Abstract
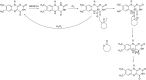
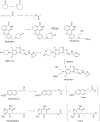
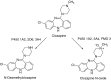
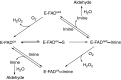
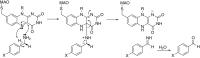
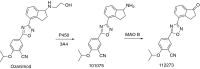
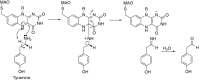
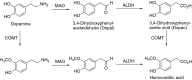
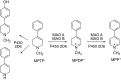
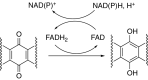
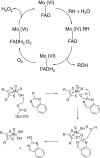
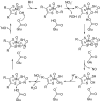
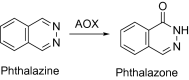
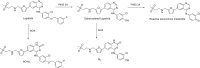
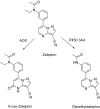
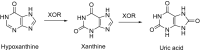
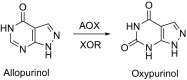
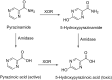
This is an overview of the metabolic reactions of drugs, natural products, physiological compounds, and other (general) chemicals catalyzed by flavin monooxygenase (FMO), monoamine oxidase (MAO), NAD(P)H quinone oxidoreductase (NQO), and molybdenum hydroxylase enzymes (aldehyde oxidase (AOX) and xanthine oxidoreductase (XOR)), including roles as substrates, inducers, and inhibitors of the enzymes. The metabolism and bioactivation of selected examples of each group (i.e., drugs, "general chemicals," natural products, and physiological compounds) are discussed. We identified a higher fraction of bioactivation reactions for FMO enzymes compared to other enzymes, predominately involving drugs and general chemicals. With MAO enzymes, physiological compounds predominate as substrates, and some products lead to unwanted side effects or illness. AOX and XOR enzymes are molybdenum hydroxylases that catalyze the oxidation of various heteroaromatic rings and aldehydes and the reduction of a number of different functional groups. While neither of these two enzymes contributes substantially to the metabolism of currently marketed drugs, AOX has become a frequently encountered route of metabolism among drug discovery programs in the past 10-15 years. XOR has even less of a role in the metabolism of clinical drugs and preclinical drug candidates than AOX, likely due to narrower substrate specificity.
Keywords: Bioactivation; Flavin-containing monooxygenase; Molybdenum hydroxylases; Monoamine oxidase; NAD(P)H quinone oxidoreductase; Natural products; Xenobiotics.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures
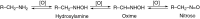
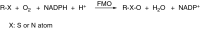

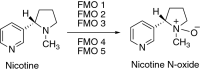
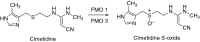

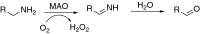
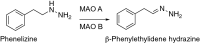
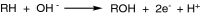
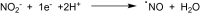
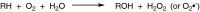
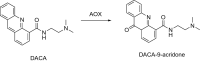
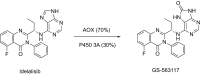
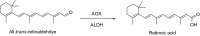


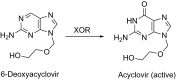
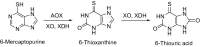
Similar articles
-
[Research advances in non-P450-mediated drug oxidative metabolism].Yao Xue Xue Bao. 2017 Jan;52(1):8-18. Yao Xue Xue Bao. 2017. PMID: 29911369 Review. Chinese.
-
Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals.Chem Res Toxicol. 2015 Jan 20;28(1):38-42. doi: 10.1021/tx500444e. Epub 2014 Dec 19. Chem Res Toxicol. 2015. PMID: 25485457 Free PMC article. Review.
-
Evolution, expression, and substrate specificities of aldehyde oxidase enzymes in eukaryotes.J Biol Chem. 2020 Apr 17;295(16):5377-5389. doi: 10.1074/jbc.REV119.007741. Epub 2020 Mar 6. J Biol Chem. 2020. PMID: 32144208 Free PMC article. Review.
-
Human Family 1-4 cytochrome P450 enzymes involved in the metabolic activation of xenobiotic and physiological chemicals: an update.Arch Toxicol. 2021 Feb;95(2):395-472. doi: 10.1007/s00204-020-02971-4. Epub 2021 Jan 18. Arch Toxicol. 2021. PMID: 33459808 Free PMC article. Review.
-
Inhibitory effects of flavonoids on molybdenum hydroxylases activity.Expert Opin Drug Metab Toxicol. 2010 Feb;6(2):133-52. doi: 10.1517/17425250903426164. Expert Opin Drug Metab Toxicol. 2010. PMID: 20095789 Review.
Cited by
-
Genomic Survey of Flavin Monooxygenases in Wild and Cultivated Rice Provides Insight into Evolution and Functional Diversities.Int J Mol Sci. 2023 Feb 20;24(4):4190. doi: 10.3390/ijms24044190. Int J Mol Sci. 2023. PMID: 36835601 Free PMC article.
-
Roles of Individual Human Cytochrome P450 Enzymes in Drug Metabolism.Pharmacol Rev. 2024 Oct 16;76(6):1104-1132. doi: 10.1124/pharmrev.124.001173. Pharmacol Rev. 2024. PMID: 39054072 Review.
-
Activity of NAD(P)H-Oxidoreductases in Ovarian Cancer.Biomedicines. 2024 May 10;12(5):1052. doi: 10.3390/biomedicines12051052. Biomedicines. 2024. PMID: 38791014 Free PMC article.
-
Copper-Nitroxyl-Catalyzed α-Oxygenation of Cyclic Secondary Amines Including Application to Late-Stage Functionalization.J Am Chem Soc. 2024 May 29;146(21):14439-14444. doi: 10.1021/jacs.4c04359. Epub 2024 May 14. J Am Chem Soc. 2024. PMID: 38743876 Free PMC article.
-
Comparative Genomics and Pathogenicity Analysis of Three Fungal Isolates Causing Barnyard Grass Blast.J Fungi (Basel). 2024 Dec 13;10(12):868. doi: 10.3390/jof10120868. J Fungi (Basel). 2024. PMID: 39728364 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

