AFM-based nanoindentation indicates an impaired cortical stiffness in the AAV-PCSK9DY atherosclerosis mouse model
- PMID: 35648220
- PMCID: PMC9393126
- DOI: 10.1007/s00424-022-02710-x
AFM-based nanoindentation indicates an impaired cortical stiffness in the AAV-PCSK9DY atherosclerosis mouse model
Abstract
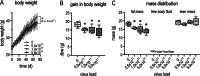
Investigating atherosclerosis and endothelial dysfunction has mainly become established in genetically modified ApoE-/- or LDL-R-/- mice transgenic models. A new AAV-PCSK9DYDY mouse model with no genetic modification has now been reported as an alternative atherosclerosis model. Here, we aimed to employ this AAV-PCSK9DY mouse model to quantify the mechanical stiffness of the endothelial surface, an accepted hallmark for endothelial dysfunction and forerunner for atherosclerosis. Ten-week-old male C57BL/6 N mice were injected with AAV-PCSK9DY (0.5, 1 or 5 × 1011 VG) or saline as controls and fed with Western diet (1.25% cholesterol) for 3 months. Total cholesterol (TC) and triglycerides (TG) were measured after 6 and 12 weeks. Aortic sections were used for atomic force microscopy (AFM) measurements or histological analysis using Oil-Red-O staining. Mechanical properties of in situ endothelial cells derived from ex vivo aorta preparations were quantified using AFM-based nanoindentation. Compared to controls, an increase in plasma TC and TG and extent of atherosclerosis was demonstrated in all groups of mice in a viral load-dependent manner. Cortical stiffness of controls was 1.305 pN/nm and increased (10%) in response to viral load (≥ 0.5 × 1011 VG) and positively correlated with the aortic plaque content and plasma TC and TG. For the first time, we show changes in the mechanical properties of the endothelial surface and thus the development of endothelial dysfunction in the AAV-PCSK9DY mouse model. Our results demonstrate that this model is highly suitable and represents a good alternative to the commonly used transgenic mouse models for studying atherosclerosis and other vascular pathologies.
Keywords: AAV-PCSK9DY mouse model; Atherosclerosis; Atomic force microscopy; Cortical stiffness; Endothelial dysfunction.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The anti-atherosclerotic effect of chronic AT1 receptor blocker treatment also depends on the ACE2/Ang(1-7)/Mas axis.Biomed Pharmacother. 2025 May;186:117990. doi: 10.1016/j.biopha.2025.117990. Epub 2025 Mar 18. Biomed Pharmacother. 2025. PMID: 40106968
-
Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9.Arterioscler Thromb Vasc Biol. 2015 Jan;35(1):50-9. doi: 10.1161/ATVBAHA.114.303617. Epub 2014 Oct 23. Arterioscler Thromb Vasc Biol. 2015. PMID: 25341796
-
A novel mouse model of diabetes, atherosclerosis and fatty liver disease using an AAV8-PCSK9-D377Y injection and dietary manipulation in db/db mice.Biochem Biophys Res Commun. 2022 Sep 24;622:163-169. doi: 10.1016/j.bbrc.2022.07.031. Epub 2022 Jul 15. Biochem Biophys Res Commun. 2022. PMID: 35868060
-
PCSK9 Biology and Its Role in Atherothrombosis.Int J Mol Sci. 2021 May 30;22(11):5880. doi: 10.3390/ijms22115880. Int J Mol Sci. 2021. PMID: 34070931 Free PMC article. Review.
-
Research progress on alternative non-classical mechanisms of PCSK9 in atherosclerosis in patients with and without diabetes.Cardiovasc Diabetol. 2020 Mar 13;19(1):33. doi: 10.1186/s12933-020-01009-4. Cardiovasc Diabetol. 2020. PMID: 32169071 Free PMC article. Review.
Cited by
-
Incidence of microvascular dysfunction is increased in hyperlipidemic mice, reducing cerebral blood flow and impairing remote memory.Front Endocrinol (Lausanne). 2024 Feb 26;15:1338458. doi: 10.3389/fendo.2024.1338458. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38469142 Free PMC article.
-
The extracellular matrix mechanics in the vasculature.Nat Cardiovasc Res. 2023 Aug;2(8):718-732. doi: 10.1038/s44161-023-00311-0. Epub 2023 Aug 10. Nat Cardiovasc Res. 2023. PMID: 39195965 Free PMC article. Review.
-
Modulation of arterial intima stiffness by disturbed blood flow.Exp Biol Med (Maywood). 2024 Jul 31;249:10090. doi: 10.3389/ebm.2024.10090. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39143955 Free PMC article.
-
Artery segmentation and atherosclerotic plaque quantification using AI for murine whole slide images stained with oil red O.Sci Rep. 2025 Apr 23;15(1):14152. doi: 10.1038/s41598-025-93967-6. Sci Rep. 2025. PMID: 40268998 Free PMC article.
References
-
- Aherrahrou R, Kulle AE, Alenina N, Werner R, Vens-Cappell S, Bader M, Schunkert H, Erdmann J, Aherrahrou Z. CYP17A1 deficient XY mice display susceptibility to atherosclerosis, altered lipidomic profile and atypical sex development. Sci Rep. 2020;10(1):8792. doi: 10.1038/s41598-020-65601-0. - DOI - PMC - PubMed
-
- Bar A, Kus K, Manterys A, Proniewski B, Sternak M, Przyborowski K, Moorlag M, Sitek B, Marczyk B, Jasztal A, Skórka T, Franczyk-Żarów M, Kostogrys RB, Chlopicki S. Vitamin K(2)-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR(-/-) mice. Vascul Pharmacol. 2019;122–123:106581. doi: 10.1016/j.vph.2019.106581. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

