Consequences of the Hsp110DE9 mutation in tumorigenesis and the 5-fluorouracil-based chemotherapy response in Msh2-deficient mice
- PMID: 35648235
- PMCID: PMC11072706
- DOI: 10.1007/s00018-022-04293-3
Consequences of the Hsp110DE9 mutation in tumorigenesis and the 5-fluorouracil-based chemotherapy response in Msh2-deficient mice
Abstract
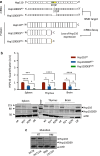
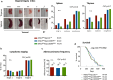
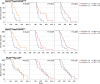
Heat shock proteins (HSPs) play oncogenic roles in human tumours. We reported a somatic inactivating mutation of HSP110 (HSP110DE9) in mismatch repair-deficient (dMMR) cancers displaying microsatellite instability (MSI) but did not assess its impact. We evaluated the impact of the Hsp110DE9 mutation on tumour development and the chemotherapy response in a dMMR knock-in mouse model (Hsp110DE9KIMsh2KO mice). The effect of the Hsp110DE9 mutation on tumorigenesis and survival was evaluated in Msh2KO mice that were null (Hsp110wt), heterozygous (Hsp110DE9KI/+), or homozygous (Hsp110DE9KI/KI) for the Hsp110DE9 mutation by assessing tumoral syndrome (organomegaly index, tumour staging) and survival (Kaplan-Meier curves). 5-Fluorouracil (5-FU), which is the backbone of chemotherapy regimens in gastrointestinal cancers and is commonly used in other tumour types but is not effective against dMMR cells in vivo, was administered to Hsp110DE9KI/KI, Hsp110DE9KI/+, and Hsp110wtMsh2KO mice. Hsp110, Ki67 (proliferation marker) and activated caspase-3 (apoptosis marker) expression were assessed in normal and tumour tissue samples by western blotting, immunophenotyping and cell sorting. Hsp110wt expression was drastically reduced or totally lost in tumours from Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice. The Hsp110DE9 mutation did not affect overall survival or tumoral syndrome in Msh2KOHsp110DE9KI/+ and Msh2KOHsp110DE9KI/KI mice but drastically improved the 5-FU response in all cohorts (Msh2KOHsp110DE9KI/KI: P5fu = 0.001; Msh2KOHsp110DE9KI/+: P5fu = 0.005; Msh2KOHsp110wt: P5fu = 0.335). Histopathological examination and cell sorting analyses confirmed major hypersensitization to 5-FU-induced death of both Hsp110DE9KI/KI and Hsp110DE9KI/+ dMMR cancer cells. This study highlights how dMMR tumour cells adapt to HSP110 inactivation but become hypersensitive to 5-FU, suggesting Hsp110DE9 as a predictive factor of 5-FU efficacy.
Keywords: 5-FU chemotherapy; Hsp110; MMR deficiency; Microsatellite instability; Mouse model.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Figures


References
-
- Svrcek M, Lascols O, Cohen R, Collura A, Jonchere V, Flejou JF, Buhard O, Duval A. MSI/MMR-deficient tumor diagnosis: Which standard for screening and for diagnosis? Diagnostic modalities for the colon and other sites: Differences between tumors. Bull Cancer. 2019;106:119–128. doi: 10.1016/j.bulcan.2018.12.008. - DOI - PubMed
-
- Boudesco C, Verhoeyen E, Martin L, et al. HSP110 sustains chronic NF-κB signaling in activated B-cell diffuse large B-cell lymphoma through MyD88 stabilization. Blood J Am Soc Hematol. 2018;132(5):510–520. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

