Brain metastases and immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 35648238
- PMCID: PMC10992555
- DOI: 10.1007/s00262-022-03224-2
Brain metastases and immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Background: The use of immune checkpoint inhibitors (ICIs) for brain metastases (BMs) from non-small cell lung cancer (NSCLC) remains debatable. This study aimed to explore the efficacy of ICIs for NSCLC with BMs. We also evaluated the effect of BMs on outcomes of ICIs.
Methods: A systematic search of PubMed, Embase, Web of Science, and Cochrane databases was conducted to identify studies where the efficacy of ICIs against BMs from NSCLC, or the association between BMs and outcomes of ICIs were evaluated. Outcomes included intracranial objective response rate (icORR), intracranial disease control rate (icDCR), systemic ORR and DCR.
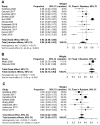
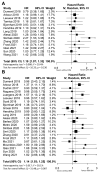
Results: Overall, 33 studies were included in this meta-analysis. The pooled icORR was 13% (95%CI 6-23%) and icDCR was 50% (95%CI 40-63%) for programmed cell death-ligand 1 (PD-L1) unselected patients with any BMs. For active BMs, pooled icORR was 15% (95%CI 6-28%) and icDCR was 47% (95% CI 36-57%). For PD-L1 ≥ 50% patients with any BMs, pooled icORR and icDCR were 68% (95%CI 57-80%) and 82% (95%CI 73-92%), respectively. Additionally, pooled systemic ORR and DCR for any BMs were 22% (95%CI 15-30%) and 41% (95%CI 18-67%), respectively. Patients with BMs had inferior progression-free survival (HR 1.19, 95%CI 1.07-1.33, P = 0.0016) and overall survival (HR 1.14, 95%CI 1.03-1.25, P = 0.011) when applying ICIs compared to those without BMs. However, no significant difference in systemic ORR between patients with and without BMs was observed (OR 0.94, 95%CI 0.72-1.20, P = 0.629).
Conclusion: ICIs may be clinically active in NSCLC patients with BMs. More effective treatments for BMs from NSCLC are needed.
Keywords: Brain metastases; Immune checkpoint inhibitor; Meta-analysis; Non-small cell lung cancer.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures



Similar articles
-
Efficacy of PD-1/L1 inhibitors in brain metastases of non-small-cell lung cancer: pooled analysis from seven randomized controlled trials.Future Oncol. 2022 Jan;18(3):403-412. doi: 10.2217/fon-2021-0795. Epub 2021 Nov 17. Future Oncol. 2022. PMID: 34787500
-
Outcome of Patients with Non-Small Cell Lung Cancer and Brain Metastases Treated with Checkpoint Inhibitors.J Thorac Oncol. 2019 Jul;14(7):1244-1254. doi: 10.1016/j.jtho.2019.02.009. Epub 2019 Feb 16. J Thorac Oncol. 2019. PMID: 30780002
-
Efficacy of PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer and brain metastases: A real-world retrospective study in China.Thorac Cancer. 2021 Nov;12(22):3019-3031. doi: 10.1111/1759-7714.14171. Epub 2021 Oct 1. Thorac Cancer. 2021. PMID: 34596346 Free PMC article.
-
The benefits and risks of CTLA4 inhibitor plus PD1/PDL1 inhibitor in stage IIIB/IV non-small cell lung cancer: A systematic analysis and meta-analysis based on randomized controlled trials.J Clin Pharm Ther. 2021 Dec;46(6):1519-1530. doi: 10.1111/jcpt.13465. Epub 2021 Jun 8. J Clin Pharm Ther. 2021. PMID: 34101874
-
The relationship between blood-based tumor mutation burden level and efficacy of PD-1/PD-L1 inhibitors in advanced non-small cell lung cancer: a systematic review and meta-analysis.BMC Cancer. 2021 Nov 13;21(1):1220. doi: 10.1186/s12885-021-08924-z. BMC Cancer. 2021. PMID: 34774004 Free PMC article.
Cited by
-
Combination of pemetrexed with bevacizumab for non-small-cell lung cancer: a meta-analysis study.J Cardiothorac Surg. 2024 Aug 2;19(1):478. doi: 10.1186/s13019-024-02975-6. J Cardiothorac Surg. 2024. PMID: 39090722 Free PMC article.
-
Association of previously irradiated stable brain metastases with outcomes of atezolizumab-treated non-small cell lung cancer: A pooled analysis of individual patient data from three randomized trials.Cancer Commun (Lond). 2024 Feb;44(2):278-281. doi: 10.1002/cac2.12512. Epub 2023 Dec 25. Cancer Commun (Lond). 2024. PMID: 38145967 Free PMC article. No abstract available.
-
A retrospective study of radiotherapy combined with immunotherapy for patients with baseline brain metastases from non-small cell lung cancer.Sci Rep. 2025 Feb 27;15(1):7036. doi: 10.1038/s41598-025-91863-7. Sci Rep. 2025. PMID: 40016281 Free PMC article.
-
A case of discordant histology and expression of programmed death ligand 1 between primary tumor and brain metastases in adenosquamous carcinoma of the lung.Thorac Cancer. 2023 Sep;14(26):2707-2711. doi: 10.1111/1759-7714.15061. Epub 2023 Aug 6. Thorac Cancer. 2023. PMID: 37545057 Free PMC article.
-
Optimal timing and sequence of combining stereotactic radiosurgery with immune checkpoint inhibitors in treating brain metastases: clinical evidence and mechanistic basis.J Transl Med. 2023 Apr 5;21(1):244. doi: 10.1186/s12967-023-04089-4. J Transl Med. 2023. PMID: 37020242 Free PMC article. Review.
References
-
- Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. - DOI - PubMed
-
- Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2006;1:205–210. doi: 10.1016/s1556-0864(15)31569-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

