Impact of IL-21-associated peripheral and brain crosstalk on the Alzheimer's disease neuropathology
- PMID: 35648273
- PMCID: PMC9160131
- DOI: 10.1007/s00018-022-04347-6
Impact of IL-21-associated peripheral and brain crosstalk on the Alzheimer's disease neuropathology
Abstract
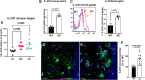
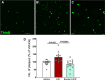
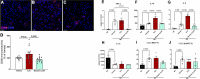
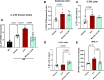
Alzheimer's disease (AD) is associated with dysregulated immune and inflammatory responses. Emerging evidence indicates that peripheral immune activation is linked to neuroinflammation and AD pathogenesis. The present study focuses on determining the role of IL-21 in the pathogenesis of AD using human samples and the 5xFAD mice model. We find that the levels of IL-21 are increased in the periphery of both humans and mice in AD. In addition, the proportions of IL-21 target cells, Tfh and B plasma cells as well as activation of monocytes is increased in PBMCs from AD and mild cognitively impaired (MCI) subjects as compared to age-matched controls, indicating immune activation. In contrast, the percentage of B1 cells that control inflammation is decreased. These changes are due to IL-21 as the expression of IL-21 receptor (IL-21R) is higher on all these cells in AD. Furthermore, treatment with recombinant IL-21 in AD mice also leads to similar alterations in Tfh, B, B1, and macrophages. The effect of IL-21 is not confined to the periphery since increased expression of IL-21R is also observed in both humans and mice hippocampus derived from the AD brains. In addition, mice injected with IL-21 display increased deposition of amyloid beta (Aβ) plaques in the brain which is reduced following anti-IL-21R antibody that blocks the IL-21 signaling. Moreover, activation of microglia was enhanced in IL-21-injected mice. In keeping with enhanced microglial activation, we also observed increased production of pro-inflammatory cytokines, IL-18 and IL-6 in IL-21-injected mice. The microglial activation and cytokines were both inhibited following IL-21R blockage. Altogether, IL-21 escalates AD pathology by enhancing peripheral and brain immune and inflammatory responses leading to increased Aβ plaque deposition. IL-21 impacts AD neuropathology by enhancing peripheral and neuronal immune activation, inflammation, and Aβ plaque deposition. Increased levels of IL-21 in the circulation of AD and MCI subjects enhances the proportions of Tfh and B plasma cells indicative of peripheral immune activation. On the other hand, the proportions of B1 cells that help reduce inflammation and clear Aβ are reduced. In addition to the periphery, IL-21 also acts on the brain via IL-21 receptor, IL-21R that displays increased expression in the hippocampi of AD and MCI subjects. IL-21 enhances the activation of microglia, induces the secretion of pro-inflammatory cytokines and deposition of Aβ plaques in the brain in AD.
Keywords: Alzheimer’s disease; B1 cells; IL-21; Neuroinflammation; Tfh.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

