Immunoglobulin and Monoclonal Antibody Therapies in Guillain-Barré Syndrome
- PMID: 35648286
- PMCID: PMC9159039
- DOI: 10.1007/s13311-022-01253-4
Immunoglobulin and Monoclonal Antibody Therapies in Guillain-Barré Syndrome
Abstract
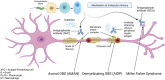
Guillain-Barré syndrome (GBS) is an acute autoimmune polyradiculoneuropathy affecting 1-2 subjects per 100,000 every year worldwide. It causes, in its classic form, symmetric weakness in the proximal and distal limb muscles with common involvement of the cranial nerves, particularly facial weakness. Respiratory function is compromised in a case in four. Randomised controlled trials have demonstrated the benefit of therapeutic plasma exchange in hastening time to recovery. Intravenous immunoglobulin was subsequently shown to be as efficacious as plasma exchange in adult subjects. In children, few trials have shown the benefit of intravenous immunoglobulin versus supportive care. Pharmacokinetic studies suggested a relationship between increase in immunoglobulin G level post-infusion and outcome, implying administration of larger doses may be beneficial in subjects with poor prognosis. However, a subsequent trial of a second dose of immunoglobulin in such subjects failed to show improved outcome, while demonstrating a higher risk of thromboembolic side-effects. Monoclonal antibody therapy has more recently been investigated for GBS, after multiple studies in animal models, with different agents and variable postulated mechanisms of action. Eculizumab, a humanised monoclonal antibody against the complement protein C5, was tested in in two randomised, double-blind, placebo-controlled phase 2 trials. Neither showed benefit versus immunoglobulins alone on disability level at 4 weeks, although one study importantly suggested possible, clinically highly relevant, late effects on normalising function. A phase 3 trial is in progress. Preliminary results of a placebo-controlled ongoing study of ANX005, a humanised recombinant antibody against C1q inhibiting the complement cascade, have been promising.
Keywords: ANX005; Eculizumab; Guillain-Barré syndrome; Immunoglobulin; Monoclonal antibody; Plasma exchange.
© 2022. The Author(s).
Figures
References
-
- Guillain G, Barré JA, Strohl A. [Radiculoneuritis syndrome with hyperalbuminosis of cerebrospinal fluid without cellular reaction. Notes on clinical features and graphs of tendon reflexes. 1916]. Ann Med Interne (Paris). 1999;150(1):24–32. - PubMed
-
- Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366(9497):1653–1666. - PubMed
-
- Jacobs BC, Rothbarth PH, van der Meché FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case-control study. Neurology. 1998;51(4):1110–1115. - PubMed
-
- Rees JH, Soudain SE, Gregson NA, Hughes RA. Campylobacter jejuni infection and Guillain-Barré syndrome. N Engl J Med. 1995;333(21):1374–1379. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous