Analysis of factors leading to early termination in glioblastoma-related clinical trials
- PMID: 35648307
- PMCID: PMC9158304
- DOI: 10.1007/s11060-022-04039-y
Analysis of factors leading to early termination in glioblastoma-related clinical trials
Abstract
Purpose: Terminated clinical trials are an inefficient use of financial, patient, and administrative resources. We reviewed ClinicalTrials.gov for completed and terminated clinical trials for glioblastoma multiforme (GBM) and compared reported characteristics of completed and terminated trials to identify factors associated with early trial termination.
Methods: ClinicalTrials.gov was queried to identify all completed and terminated GBM-related clinical trials. Trial characteristics were examined and the reason for trial termination was determined. Univariate analysis by Pearson's chi-square and a multivariate logistic regression were performed to identify independent predictors of early trial termination.
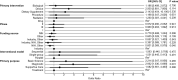
Results: We identified 886 completed and terminated GBM-related trials between 2003 and 2020. Of these, 175 (19.8%) were terminated prior to completion. The most common reason for termination was participant accrual difficulties, accounting for 63 (36.0%) terminated trials. Trial termination was associated with trials that reported a primary purpose of diagnosis relative to treatment (OR = 2.952, p = 0.001).
Conclusion: Early termination of clinical trials investigating interventions for the treatment of GBM is associated with diagnostic trials relative to therapeutic trials. Patient accrual difficulties are the most commonly identified reason for early trial termination. Predictors of trial termination should be considered when designing GBM-related clinical trials to minimize the odds of early trial termination.
Keywords: Clinical trial termination; Clinical trials; Glioblastoma; Glioblastoma trials; Glioma.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no relevant competing interests to disclose.
Figures
References
-
- Bagley SJ, Kothari S, Rahman R, Lee EQ, Dunn GP, Galanis E, Chang SM, Nabors LB, Ahluwalia MS, Stupp R, Mehta MP, Reardon DA, Grossman SA, Sulman EP, Sampson JH, Khagi S, Weller M, Cloughesy TF, Wen PY, Khasraw M. Glioblastoma clinical trials: current landscape and opportunities for improvement. Clin Cancer Res. 2022;28(4):594–602. doi: 10.1158/1078-0432.CCR-21-2750. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

