The humoral immune response more than one year after SARS-CoV-2 infection: low detection rate of anti-nucleocapsid antibodies via Euroimmun ELISA
- PMID: 35648370
- PMCID: PMC9159036
- DOI: 10.1007/s15010-022-01830-x
The humoral immune response more than one year after SARS-CoV-2 infection: low detection rate of anti-nucleocapsid antibodies via Euroimmun ELISA
Abstract
Purpose: Antibody assays against SARS-CoV-2 are used in sero-epidemiological studies to estimate the proportion of a population with past infection. IgG antibodies against the spike protein (S-IgG) allow no distinction between infection and vaccination. We evaluated the role of anti-nucleocapsid-IgG (N-IgG) to identify individuals with infection more than one year past infection.
Methods: S- and N-IgG were determined using the Euroimmun enzyme-linked immunosorbent assay (ELISA) in two groups: a randomly selected sample from the population of Stuttgart, Germany, and individuals with PCR-proven SARS-CoV-2 infection. Participants were five years or older. Demographics and comorbidities were registered from participants above 17 years.
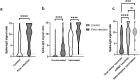
Results: Between June 15, 2021 and July 14, 2021, 454 individuals from the random sample participated, as well as 217 individuals with past SARS-CoV-2 infection. Mean time from positive PCR test result to antibody testing was 458.7 days (standard deviation 14.6 days) in the past infection group. In unvaccinated individuals, the seroconversion rate for S-IgG was 25.5% in the random sample and 75% in the past infection group (P = < 0.001). In vaccinated individuals, the mean signal ratios for S-IgG were higher in individuals with prior infection (6.9 vs 11.2; P = < 0.001). N-IgG were only detectable in 17.1% of participants with past infection. Predictors for detectable N-IgG were older age, male sex, fever, wheezing and in-hospital treatment for COVID-19 and cardiovascular comorbidities.
Conclusion: N-IgG is not a reliable marker for SARS-CoV-2 infection after more than one year. In future, other diagnostic tests are needed to identify individuals with past natural infection.
Keywords: Antibody; COVID-19; Nucleocapsid; Seroprevalence; Spike.
© 2022. The Author(s).
Conflict of interest statement
All other authors declare no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

