Laminin N-terminus α31 expression during development is lethal and causes widespread tissue-specific defects in a transgenic mouse model
- PMID: 35648586
- PMCID: PMC9328196
- DOI: 10.1096/fj.202002588RRR
Laminin N-terminus α31 expression during development is lethal and causes widespread tissue-specific defects in a transgenic mouse model
Abstract
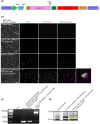
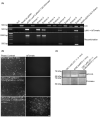
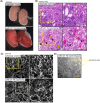
Laminins (LMs) are essential components of all basement membranes where they regulate an extensive array of tissue functions. Alternative splicing from the laminin α3 gene produces a non-laminin but netrin-like protein, Laminin N terminus α31 (LaNt α31). LaNt α31 is widely expressed in intact tissue and is upregulated in epithelial cancers and during wound healing. In vitro functional studies have shown that LaNt α31 can influence numerous aspects of epithelial cell behavior via modifying matrix organization, suggesting a new model of laminin auto-regulation. However, the function of this protein has not been established in vivo. Here, a mouse transgenic line was generated using the ubiquitin C promoter to drive inducible expression of LaNt α31. When expression was induced at embryonic day 15.5, LaNt α31 transgenic animals were not viable at birth, exhibiting localized regions of erythema. Histologically, the most striking defect was widespread evidence of extravascular bleeding across multiple tissues. Additionally, LaNt α31 transgene expressing animals exhibited kidney epithelial detachment, tubular dilation, disruption of the epidermal basal cell layer and of the hair follicle outer root sheath, and ~50% reduction of cell numbers in the liver, associated with depletion of hematopoietic erythrocytic foci. These findings provide the first in vivo evidence that LaNt α31 can influence tissue morphogenesis.
Keywords: basement membrane; development; laminin; netrin.
© 2022 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures



Similar articles
-
Laminin N-terminus α31 protein distribution in adult human tissues.PLoS One. 2020 Dec 2;15(12):e0239889. doi: 10.1371/journal.pone.0239889. eCollection 2020. PLoS One. 2020. PMID: 33264294 Free PMC article.
-
Differential Distribution of Laminin N-Terminus α31 Across the Ocular Surface: Implications for Corneal Wound Repair.Invest Ophthalmol Vis Sci. 2018 Aug 1;59(10):4082-4093. doi: 10.1167/iovs.18-24037. Invest Ophthalmol Vis Sci. 2018. PMID: 30098195 Free PMC article.
-
Laminin N-terminus α31 is upregulated in invasive ductal breast cancer and changes the mode of tumour invasion.PLoS One. 2022 Mar 1;17(3):e0264430. doi: 10.1371/journal.pone.0264430. eCollection 2022. PLoS One. 2022. PMID: 35231053 Free PMC article.
-
Laminins in lung development.Exp Lung Res. 1997 Mar-Apr;23(2):119-29. doi: 10.3109/01902149709074025. Exp Lung Res. 1997. PMID: 9088922 Review.
-
Laminin isoforms and lung development: all isoforms are not equal.Dev Biol. 2006 Jun 15;294(2):271-9. doi: 10.1016/j.ydbio.2006.03.032. Epub 2006 Apr 3. Dev Biol. 2006. PMID: 16643883 Review.
Cited by
-
Laminin Polymerization and Inherited Disease: Lessons From Genetics.Front Genet. 2021 Aug 12;12:707087. doi: 10.3389/fgene.2021.707087. eCollection 2021. Front Genet. 2021. PMID: 34456976 Free PMC article. Review.
-
A Novel Transcriptome Approach to the Investigation of the Molecular Pathology of Vitreous and Retinal Detachment.Genes (Basel). 2022 Oct 18;13(10):1885. doi: 10.3390/genes13101885. Genes (Basel). 2022. PMID: 36292771 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

