Co-component signal transduction systems: Fast-evolving virulence regulation cassettes discovered in enteric bacteria
- PMID: 35648808
- PMCID: PMC9214523
- DOI: 10.1073/pnas.2203176119
Co-component signal transduction systems: Fast-evolving virulence regulation cassettes discovered in enteric bacteria
Abstract
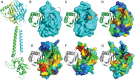
Bacterial signal transduction systems sense changes in the environment and transmit these signals to control cellular responses. The simplest one-component signal transduction systems include an input sensor domain and an output response domain encoded in a single protein chain. Alternatively, two-component signal transduction systems transmit signals by phosphorelay between input and output domains from separate proteins. The membrane-tethered periplasmic bile acid sensor that activates the Vibrio parahaemolyticus type III secretion system adopts an obligate heterodimer of two proteins encoded by partially overlapping VtrA and VtrC genes. This co-component signal transduction system binds bile acid using a lipocalin-like domain in VtrC and transmits the signal through the membrane to a cytoplasmic DNA-binding transcription factor in VtrA. Using the domain and operon organization of VtrA/VtrC, we identify a fast-evolving superfamily of co-component systems in enteric bacteria. Accurate machine learning–based fold predictions for the candidate co-components support their homology in the twilight zone of rapidly evolving sequences and provide mechanistic hypotheses about previously unrecognized lipid-sensing functions.
Keywords: co-component signal transduction system; enteric bacteria; protein sequence evolution; protein structure prediction; virulence transcription regulation.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

