Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q
- PMID: 35648810
- PMCID: PMC9554345
- DOI: 10.1126/scitranslmed.abi8593
Reversal of synapse loss in Alzheimer mouse models by targeting mGluR5 to prevent synaptic tagging by C1Q
Abstract
Microglia-mediated synaptic loss contributes to the development of cognitive impairments in Alzheimer's disease (AD). However, the basis for this immune-mediated attack on synapses remains to be elucidated. Treatment with the metabotropic glutamate receptor 5 (mGluR5) silent allosteric modulator (SAM), BMS-984923, prevents β-amyloid oligomer-induced aberrant synaptic signaling while preserving physiological glutamate response. Here, we show that oral BMS-984923 effectively occupies brain mGluR5 sites visualized by [18F]FPEB positron emission tomography (PET) at doses shown to be safe in rodents and nonhuman primates. In aged mouse models of AD (APPswe/PS1ΔE9 overexpressing transgenic and AppNL-G-F/hMapt double knock-in), SAM treatment fully restored synaptic density as measured by [18F]SynVesT-1 PET for SV2A and by histology, and the therapeutic benefit persisted after drug washout. Phospho-TAU accumulation in double knock-in mice was also reduced by SAM treatment. Single-nuclei transcriptomics demonstrated that SAM treatment in both models normalized expression patterns to a far greater extent in neurons than glia. Last, treatment prevented synaptic localization of the complement component C1Q and synaptic engulfment in AD mice. Thus, selective modulation of mGluR5 reversed neuronal gene expression changes to protect synapses from damage by microglial mediators in rodents.
Figures

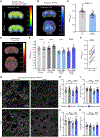




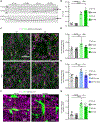
Comment in
-
Restoring synapses in AD models.Nat Rev Neurol. 2022 Aug;18(8):453. doi: 10.1038/s41582-022-00691-0. Nat Rev Neurol. 2022. PMID: 35725818 No abstract available.
References
-
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 14, 535–562 (2018). - PMC - PubMed
-
- De Strooper B, Karran E, The cellular phase of Alzheimer's disease. Cell 164, 603–615 (2016). - PubMed
-
- McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, Fagan AM, Holtzman DM, Cairns NJ, Goate AM, Marcus DS, Morris JC, Paumier K, Xiong C, Allegri R, Berman SB, Klunk W, Noble J, Ringman J, Ghetti B, Farlow M, Sperling RA, Chhatwal J, Salloway S, Graff-Radford NR, Schofield PR, Masters C, Rossor MN, Fox NC, Levin J, Jucker M, Bateman RJ; Dominantly Inherited Alzheimer Network, Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 91, e1295–e1306 (2018). - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

