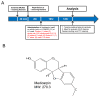
Medicarpin isolated from Radix Hedysari ameliorates brain injury in a murine model of cerebral ischemia
- PMID: 35649147
- PMCID: PMC9931010
- DOI: 10.38212/2224-6614.3377
Medicarpin isolated from Radix Hedysari ameliorates brain injury in a murine model of cerebral ischemia
Abstract
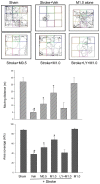
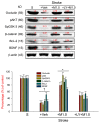
The development of effective post-stroke therapy is highly demanded. Medicarpin is a key active component of a famous Chinese herbal prescription used for post-stroke treatment in Taiwan; however, little is known about its biological effects and mechanisms of action. Herein, we implemented a murine model of cerebral ischemic/reperfusional injury-related stroke to elucidate medicarpin's neuroprotective effect. In male ICR mice 24 h after stroke induction, treatment with medicarpin (0.5 and 1.0 mg/kg, i.v.) markedly enhanced the survival rates, improved moving distance and walking area coverage, reduced brain infarction, and preserved the blood-brain barrier, supporting medicarpin's protective effect on stroke-induced injury. Immunohistochemistry analysis further revealed that medicarpin treatment decreased the expression/activation of p65NF-κB and caspase 3, especially near the infarct cortex, while promoting the expression of neurogenesis-associated proteins, including doublecortin (DCX), brain-derived neurotrophic factor (BDNF), and tyrosine receptor kinase B (TrkB). These changes of expression levels were accompanied by GSK-3 inactivation and β-catenin upregulation. Notably, pretreatment with LY294002, a PI3K inhibitor, abolished the aforementioned beneficial effects of medicarpin, illustrating an essential role of PI3K/Akt activation in medicarpin's neuroprotective and reparative activities. In vitro studies revealed that medicarpin displayed strong anti-inflammatory activity by reducing nitric oxide (NO) production in lipopolysaccharide-stimulated microglial cells (BV2) with an IC50 around 5 ±1 (μM) and anti-apoptotic activity in neuronal cells (N2A) subjected to oxygen-glucose deprivation with an IC50 around 13 ± 2 (μM). Collectively, this is the first report to demonstrate that medicarpin, isolated from Radix Hedysari, ameliorates ischemic brain injury through its anti-inflammatory microglia/NO), anti-apoptotic (neuronal cells/OGD) and neuroprotective effects by activating the PI3K/Akt-dependent GSK-3 inactivation for upregulating β-catenin, which in turn decreases the expression/activation of p65NF-κB and caspase 3 and promotes the expression of neurogenic (DCX, BDNF, TrkB) and neuroprotective (Bcl2) factors in the brain.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures












References
-
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11. - PubMed
-
- Zhou Z, Lu J, Liu WW, Manaenko A, Hou X, Mei Q, et al. Advances in stroke pharmacology. Pharmacol Ther. 2018;191:23–42. - PubMed
-
- Wang YH, Chern CM, Liou KT, Kuo YH, Shen YC. Ergostatrien-7,9(11),22-trien-3beta-ol from Antrodia camphorata ameliorates ischemic stroke brain injury via downregulation of p65NF-kappa-B and caspase 3, and activation of Akt/GSK3/catenin-associated neurogenesis. Food Funct. 2019;10:4725–38. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
