Endocannabinoids, Anandamide and 2-Arachidonoylglycerol, as Prognostic Markers of Sepsis Outcome and Complications
- PMID: 35649233
- PMCID: PMC10589499
- DOI: 10.1089/can.2022.0046
Endocannabinoids, Anandamide and 2-Arachidonoylglycerol, as Prognostic Markers of Sepsis Outcome and Complications
Abstract
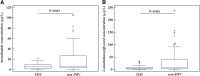
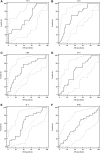
Background: One of the major challenges in improving sepsis care is early prediction of sepsis complications. The endocannabinoid system has been intensely studied in recent years; however, little is known about its role in sepsis in humans. This study aimed to assess the prognostic role of endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), as early predictors of mortality, invasive mechanical ventilation (IMV) requirement, and length of stay (LOS) in patients with sepsis. Materials and Methods: In total, 106 patients with confirmed sepsis were enrolled in this study. The patients were divided into groups according to mortality outcome (survival, N=53; nonsurvival, N=53), IMV requirement (IMV group, N=26; non-IMV group, N=80), and LOS (LOS <10 days, N=59; LOS ≥10 days, N=47). Patients' clinical status was assessed along with laboratory biomarkers as well as AEA and 2-AG concentration measurements early on admission to emergency units. AEA and 2-AG levels were measured by enzyme-linked immunosorbent assay (ELISA) using an ELISA processor, EtiMax 3000 (DiaSorin, Saluggia, Italy). The predictive value of AEA and 2-AG for the studied sepsis outcomes and complications was analyzed using univariate and multivariate analyses and receiver operating characteristic (ROC) curve analysis. Results: Two endocannabinoids showed no significant difference between survivors and nonsurvivors, although an AEA concentration <7.16 μg/L predicted mortality outcome with a sensitivity of 57% (95% confidence interval [CI] 42-71) and specificity of 80% (95% CI 66-91). AEA concentrations ≤17.84 μg/L predicted LOS ≥10 days with sensitivity of 98% (95% CI 89-100) and specificity of 34% (95% CI 22-47). When analyzing IMV requirement, levels of AEA and 2-AG were significantly lower within the IMV group compared with the non-IMV group (5.94 μg/L [2.04-9.44] and 6.70 μg/L [3.50-27.04], p=0.043, and 5.68 μg/L [2.30-8.60] and 9.58 μg/L [4.83-40.05], p=0.002, respectively). The 2-AG showed the best performance for IMV requirement prediction, with both sensitivity and specificity of 69% (p<0.001). Endocannabinoid AEA was an independent risk factor of LOS ≥10 days (odds ratio [OR] 23.59; 95% CI 3.03-183.83; p=0.003) and IMV requirement in sepsis (OR 0.79; 95% CI, 0.67-0.93; p=0.004). Conclusion: Low AEA concentration is a prognostic factor of hospital LOS longer than 10 days. Lower AEA and 2-AG concentrations obtained at the time of admission to the hospital are predictors of IMV requirement.
Keywords: 2-arachidonoylglycerol; anandamide; endocannabinoids; invasive mechanical ventilation; prognosis; sepsis.
Conflict of interest statement
No competing financial interests exist.
Figures
References
-
- Luchicchi A, Pistis A. Anandamide and 2-arachidonoylglycerol: pharmacological properties, functional features, and emerging specificities of the two major endocannabinoids. Mol Neurobiol. 2012;46:374–392. - PubMed
-
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

