Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases
- PMID: 35649411
- PMCID: PMC9271637
- DOI: 10.1016/j.medj.2022.05.002
Cross-tissue, single-cell stromal atlas identifies shared pathological fibroblast phenotypes in four chronic inflammatory diseases
Abstract
Background: Pro-inflammatory fibroblasts are critical for pathogenesis in rheumatoid arthritis, inflammatory bowel disease, interstitial lung disease, and Sjögren's syndrome and represent a novel therapeutic target for chronic inflammatory disease. However, the heterogeneity of fibroblast phenotypes, exacerbated by the lack of a common cross-tissue taxonomy, has limited our understanding of which pathways are shared by multiple diseases.
Methods: We profiled fibroblasts derived from inflamed and non-inflamed synovium, intestine, lungs, and salivary glands from affected individuals with single-cell RNA sequencing. We integrated all fibroblasts into a multi-tissue atlas to characterize shared and tissue-specific phenotypes.
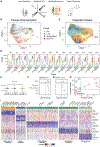
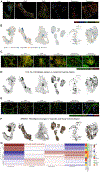
Findings: Two shared clusters, CXCL10+CCL19+ immune-interacting and SPARC+COL3A1+ vascular-interacting fibroblasts, were expanded in all inflamed tissues and mapped to dermal analogs in a public atopic dermatitis atlas. We confirmed these human pro-inflammatory fibroblasts in animal models of lung, joint, and intestinal inflammation.
Conclusions: This work represents a thorough investigation into fibroblasts across organ systems, individual donors, and disease states that reveals shared pathogenic activation states across four chronic inflammatory diseases.
Funding: Grant from F. Hoffmann-La Roche (Roche) AG.
Keywords: Foundational research; Sjögren's syndrome; atlas; fibroblasts; inflammation; integration; interstitial lung disease; rheumatoid arthritis; scRNA-seq; stromal; ulcerative colitis.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.Y.K. is a member of the advisory board for Cell Reports Medicine. In disclosures unrelated to this work, E.Y.K. is a member of the Steering Committees for and receives no financial renumeration from NCT04409834 (Prevention of arteriovenous thrombotic events in critically ill COVID-19 patients, TIMI group) and REMAP-CAP ACE2 renin-angiotensin system (RAS) modulation domain. E.Y.K. receives unrelated research funding from Bayer AG. In the past, E.Y.K. received unrelated research funding from Windtree Therapeutics. T.T. receives unrelated support from the Zoll Foundation. K.W. is a consultant to Mestag and Gilead Sciences and reports grant support from Gilead Sciences. S.R. is a scientific advisor for Rheos Medicines, Janssen, and Pfizer and a founder of Mestag, Inc. Y.J. and H.A. receive unrelated support from Bayer AG. M.B.B. is a consultant to GSK and 4FO Ventures and a founder of Mestag Therapeutics. M.L.S. receives unrelated research funding and institution consulting fees from Genentech, institution consulting fees from Lilly, research funding from Bristol Myers Squibb, and personal consulting fees from GV20 Therapeutics. M.C. is a co-founder of Mestag Therapeutics and obtains grant funding from and has consulted for Hoffman La-Roche. S.B. has provided paid consultancy services regarding Sjögren’s syndrome clinical trial design for the following companies in the past 3 years: Abbvie, AstraZeneca, BMS, Galapagos, Novartis, and Resolve Pharma. A.F. has received personal renumerations from Abbvie, Roche, and Janssen in the last 2 years and institutional research funding from Roche, UCB, Nascient, Mestag, GSK, and Janssen. A.P.F. and K.G.L. reported being employees of F. Hoffmann-La Roche (Roche) AG.
Figures





References
-
- Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, Goodman SM, Tabechian D, Hughes LB, Salomon-Escoto K, et al. (2019). Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol 20, 928–942. 10.1038/s41590-019-0378-1. - DOI - PMC - PubMed
-
- Habermann AC, Gutierrez AJ, Bui LT, Yahn SL, Winters NI, Calvi CL, Peter L, Chung MI, Taylor CJ, Jetter C, et al. (2020). Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci. Adv 6, eaba1972. 10.1126/sciadv.aba1972. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- P01 AI148102/AI/NIAID NIH HHS/United States
- MR/S025308/1/MRC_/Medical Research Council/United Kingdom
- MR/V001949/1/MRC_/Medical Research Council/United Kingdom
- T32 GM007753/GM/NIGMS NIH HHS/United States
- 22710/VAC_/Versus Arthritis/United Kingdom
- T32 GM144273/GM/NIGMS NIH HHS/United States
- 22949/VAC_/Versus Arthritis/United Kingdom
- R01 AR063759/AR/NIAMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- K08 AR077037/AR/NIAMS NIH HHS/United States
- R01 AI049313/AI/NIAID NIH HHS/United States
- 22253/VAC_/Versus Arthritis/United Kingdom
- U01 HG012009/HG/NHGRI NIH HHS/United States
- 22658/VAC_/Versus Arthritis/United Kingdom
- UC2 AR081023/AR/NIAMS NIH HHS/United States
- U19 AI111224/AI/NIAID NIH HHS/United States
- MR/S035850/1/MRC_/Medical Research Council/United Kingdom
- K01 AR078355/AR/NIAMS NIH HHS/United States
- U01 HG009379/HG/NHGRI NIH HHS/United States
- R01 AR063709/AR/NIAMS NIH HHS/United States
- UH2 AR067677/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

