Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India
- PMID: 35649613
- PMCID: PMC9160584
- DOI: 10.1136/bmjopen-2021-059540
Hydroxychloroquine plus personal protective equipment versus personal protective equipment alone for the prevention of laboratory-confirmed COVID-19 infections among healthcare workers: a multicentre, parallel-group randomised controlled trial from India
Abstract
Objectives: To determine whether hydroxychloroquine when used with personal protective equipment reduces the proportion of laboratory-confirmed COVID-19 among healthcare workers in comparison to the use of personal protective equipment alone.
Design: Multicentre, parallel-group, open-label randomised trial. Enrolment started on 29 June 2020 and stopped on 4 February 2021. Participants randomised in HydrOxychloroquine Prophylaxis Evaluation were followed for 6 months.
Setting: 9 hospitals across India.
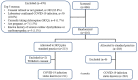
Participants: Healthcare workers in an environment with exposure to COVID-19 were randomised in a 1:1 ratio to hydroxychloroquine plus use of personal protective equipment or personal protective equipment alone. 886 participants were screened and 416 randomised (213 hydroxychloroquine arm and 203 personal protective equipment).
Intervention: Participants in intervention arm received 800 mg of hydroxychloroquine on day of randomisation and then 400 mg once a week for 12 weeks in addition to the use of personal protective equipment. In the control arm, participants continued to use personal protective equipment alone.
Main outcome: Proportion of laboratory-confirmed COVID-19 in the 6 months after randomisation.
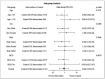
Results: Participants were young (mean age 32.1 years, SD 9.1 years) with low-comorbid burden. 47.4% were female. In the 6 months after randomisation (primary analysis population=413), 11 participants assigned to the hydroxychloroquine group and 12 participants assigned to the standard practice group met the primary endpoint (5.2% vs 5.9%; OR 0.85, 95% CI 0.35 to 2.07, p=0.72). There was no heterogeneity of treatment effect in any prespecified subgroup. There were no significant differences in the secondary outcomes. The adverse event rates were 9.9% and 6.9% in the hydroxychloroquine and standard practice arms, respectively. There were no serious adverse events in either group.
Conclusions and relevance: Hydroxychloroquine along with personal protective equipment was not superior to personal protective equipment alone on the proportion of laboratory-confirmed COVID-19. Definitive conclusions are precluded as the trial stopped early for futility, and hence was underpowered.
Trial registration number: CTRI/2020/05/025067.
Keywords: COVID-19; clinical pharmacology; public health.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: OJ reports being a member of the WHO R&D Blueprint Safety Monitoring Team, ACT Accelerator—R&D Digital Health working group and COVID-19 Clinical Research Coalition data sharing working group.
Figures
References
-
- WHO , 2021. Available: https://covid19.who.int/ [Accessed 13 Sep 2021].
-
- WHO , 2021. Available: https://covid19.who.int/region/searo/country/in [Accessed 13 Sep 2021].
-
- WHO , 2021. Available: https://www.who.int/csr/sars/country/table2004_04_21/en/ [Accessed 13 Sep 2021].
-
- WHO , 2021. Available: http://applications.emro.who.int/docs/EMCSR246E.pdf?ua=1 [Accessed 13 Sep 2021].
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
