Theta but not beta activity is modulated by freedom of choice during action selection
- PMID: 35650241
- PMCID: PMC9160249
- DOI: 10.1038/s41598-022-13318-7
Theta but not beta activity is modulated by freedom of choice during action selection
Abstract
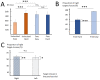
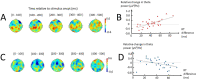
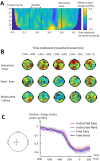
Large-scale neurophysiological markers of action competition have been almost exclusively investigated in the context of instructed choices, hence it remains unclear whether these markers also apply to free choices. This study aimed to compare the specific brain dynamics underlying instructed and free decisions. Electroencephalography (EEG) was recorded while 31 participants performed a target selection task; the choice relied either on stimulus-response mappings (instructed) or on participants' preferences (free). Choice difficulty was increased by introducing distractors in the informative stimulus in instructed choices, and by presenting targets with similar motor costs in free choices. Results revealed that increased decision difficulty was associated with higher reaction times (RTs) in instructed choices and greater choice uncertainty in free choices. Midfrontal EEG theta (4-8 Hz) power increased with difficulty in instructed choices, but not in free choices. Although sensorimotor beta (15-30 Hz) power was correlated with RTs, it was not significantly influenced by choice context or difficulty. These results suggest that midfrontal theta power may specifically increase with difficulty in externally-driven choices, whereas sensorimotor beta power may be predictive of RTs in both externally- and internally-driven choices.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Freedom of choice boosts midfrontal theta power during affective feedback processing of goal-directed actions.Biol Psychol. 2023 Oct;183:108659. doi: 10.1016/j.biopsycho.2023.108659. Epub 2023 Aug 11. Biol Psychol. 2023. PMID: 37572945
-
When the brain changes its mind: flexibility of action selection in instructed and free choices.Cereb Cortex. 2009 Oct;19(10):2352-60. doi: 10.1093/cercor/bhn252. Epub 2009 Feb 11. Cereb Cortex. 2009. PMID: 19211661 Free PMC article.
-
My choice, my actions: self-determination, not instrumental value of outcomes enhances outcome monitoring during learning.Cereb Cortex. 2024 Aug 1;34(8):bhae325. doi: 10.1093/cercor/bhae325. Cereb Cortex. 2024. PMID: 39118215
-
Learning to Synchronize: Midfrontal Theta Dynamics during Rule Switching.J Neurosci. 2021 Feb 17;41(7):1516-1528. doi: 10.1523/JNEUROSCI.1874-20.2020. Epub 2020 Dec 11. J Neurosci. 2021. PMID: 33310756 Free PMC article.
-
Cortical beta-band power modulates with uncertainty in effector selection during motor planning.J Neurophysiol. 2021 Dec 1;126(6):1891-1902. doi: 10.1152/jn.00198.2021. Epub 2021 Nov 3. J Neurophysiol. 2021. PMID: 34731060
Cited by
-
Cognitive training and retest learning effects on theta and alpha power in older and young adults: A perspective on the crunch hypothesis and the STAC-R model.Int J Clin Health Psychol. 2025 Apr-Jun;25(2):100568. doi: 10.1016/j.ijchp.2025.100568. Epub 2025 Apr 17. Int J Clin Health Psychol. 2025. PMID: 40292420 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

